Author(s):
Isaac Melamed*, Lacey Robinson and Melinda Heffron
IMMUNOe International Research Centers, Centennial, CO, USA
Received: 10 April, 2017; Accepted: 03 May, 2017; Published: 04 May, 2017
Isaac Melamed, ImmunoE Health Centers, 6801 South Yosemite Street, Centennial, USA, Tel: 303-224-4678; 720-273-2863; Fax: 303-224-4699; E-mail:
Melamed I, Robinson L, Heffron M (2017) The Benefit of Montelukast in Atopic Dermatitis Induced by Food Allergies. Glob J Allergy 3(1): 013-018. DOI: 10.17352/2455-8141.000018
© 2017 Patella V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Cysteinyl leukotriene levels are elevated in patients with atopic dermatitis, which can lead to eosinophilic infiltration of the gastrointestinal tract.
Objective: We examined the role that montelukast (a leukotriene receptor antagonist) might play in improving symptoms of atopic dermatitis induced by food allergies.
Methods: We conducted a randomized, double-blind, placebo-controlled, parallel group study in 20 children, aged 1 to 8 years, with 4 study visits every 3 weeks for 9 weeks. Primary inclusion criteria consisted of: 1) positive reactivity to food (indicated by skin or RAST test); 2) 10–25 % body area affected with atopic dermatitis; and 3) gastrointestinal symptoms. Liquid cetirizine and 1% hydrocortisone cream were both given as rescue medications for atopic dermatitis flare-ups. Pruritis and atopic dermatitis flare-up scores were used to collect clinical data. Laboratory values for nerve growth factor were evaluated pre- and post-treatment.
Results: Our main endpoints were the effects of montelukast on the clinical presentation of atopic dermatitis. When comparing the treatment group to placebo, we noted a significant reduction in the pruritis score (p=0.002); a trend toward a reduction in the use of rescue medication (cetirizine: p=0.056; hydrocortisone cream: p=0.056); and a reduction in the level of nerve growth factor; mean values: placebo=3.06 to montelukast=2.59.
Conclusion: The inflammatory pathway triggered by food allergies that may lead to atopic dermatitis can be modulated with montelukast. Furthermore, nerve growth factor may play a role in the pathogenesis of atopic dermatitis and montelukast may modify this pathway
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disorder [1]. The inflammatory process seen in AD is characterized by a mixture of immunological and pharmacological abnormalities that can be detected in the serum and at the cellular level. Included in the immune histology of AD skin are infiltrates of activated T-cells, dendrite presenting cells, eosinophils, and mast cells [2].
Commencement of the immune cascade can result from multiple activators/activations, including allergens (food or environmental), infectious pathogens, and numerous other distinctive triggers (eg, stress or anxiety) [3]. The cascade involves a series of reactions that culminate in different biologic responses, including entry into the cell cycle or cell death. While programmed cell death, or apoptosis, can be triggered under a variety of circumstances, the mechanism by which cells are directed to cell cycle progression as opposed to apoptosis is not well understood [4]. In recent years, increasing evidence has suggested that regulatory T-cells are involved in various skin diseases and that they play a pivotal role in the etiology of AD [5]. These roles are complex and may involve various mechanisms, showing that the immune response leading to inflammation due to a failure of apoptosis is a crucial factor [6,7].
Cysteinyl leukotrienes (Cys-LTs) are the products of arachidonic acid metabolism and are released from various cells, including mast cells, basophils, and eosinophils; they have long been known to be potent mediators of allergic inflammation. The secretion of these products (eicosanoids) and the occupation of the Cys-LT receptors have been correlated with the pathophysiology of both asthma and allergic rhinitis in numerous studies. It has recently been suggested that Cys-LTs may also play a role in food allergies and their subsequent immune response [8-12].
Recent studies have shown that some food allergens can increase the levels of Cys-LTs [8-12]. These studies suggested that leukotriene receptor antagonists could potentially interfere with the inflammatory cascade induced by food allergens. A recent study by Adamek-Guzik et al found that the levels of Cys-LTs in patients with AD were significantly higher during skin flare-up compared to the remission phase [8]. It also noted that the clinical severity of skin lesions correlated with increased levels of Cys-LTs.
Other studies concur with the use of leukotriene receptor antagonists for AD patients [7,12]. These studies show a correlation between the occurrence of AD in patients with known food intolerances. In the presence of food allergens, increased Cys-LT production by peripheral leukocytes (specifically eosinophils) was observed in the majority of patients. Eosinophilic involvement in the pathogenesis of AD has previously been established. The immune response in AD causes an increase in the amount of Cys-LTs brought about by food intolerance. These studies also suggest the use of leukotriene receptor antagonists for reduction in the severity of AD for patients with food allergies.
The importance of eosinophilic involvement in the inflammatory response is also found to occur in gastrointestinal (GI) disorders [10,11]. The infiltration of eosinophils into the GI tract is seen in eosinophilic gastroenteritis (EG), which causes severe tissue damage to the gut. Food allergies are a triggering factor in the allergic response, thereby causing the accumulation of eosinophils in the tissues of the GI tract. EG and AD are strongly associated with known food allergies in about 70% of the cases.
In vitro studies examined the effect of leukotriene B4 (LTB4) antagonists on eosinophilic infiltration in the gut and skin [13], BALB/c mice received an oral challenge to induce eosinophilic infiltration into the gut and skin. Peak infiltration occurred at 6 hours (gut) and 12 hours (skin) after the challenge. If an intraperitoneal dosage of a LTB4 antagonist was administered before the oral challenge, a significant inhibition of eosinophilic infiltration was noted. A reduction of 53.3% in the skin and 73.3% in the gut was measured. Although these results were not conducted in humans, they point to the advisability of using a leukotriene inhibitor to prevent eosinophilic infiltration in the skin and gut in cases of known food allergies.
Considerable evidence suggests a crosstalk between the nervous and immune systems. Previously, we investigated the role of nerve growth factor (NGF) in this crosstalk and showed an active role for NGF in B-cell cytoskeletal signaling [14-19]. It has been suggested that NGF plays a role in the inflammatory process and in tissue repair [20]. Both skin and lung fibroblasts produce NGF and express tyrosine kinase receptor under basal conditions. In cutaneous inflammatory responses, the expression of NGF receptor (TrkA) is reduced following an acute inflammatory stimulus and also in association with chronic inflammatory dermatitis [21,22], suggesting that NGF plays an active role in the pathogenesis of AD [23].
Leukotriene receptor antagonists are specific in blocking the Cys-LT receptor, thereby preventing the immune cascade response. Montelukast, the active ingredient in the product Singulair®, has been shown to be a selective and potent leukotriene receptor antagonist and has been studied extensively for its use in the reduction or prevention of airway inflammation and allergic rhinitis. As leukotriene B4 (LTB4) antagonists may reduce eosinophilic infiltration into the gut and skin, we formed a hypothesis regarding the role of food allergens in the induction of eosinophilic activation and their contribution to dermatological symptoms.
Our hypothesis for this research study was that NGF may play a role in the inflammatory process and that montelukast would possibly:
1. Downregulate the inflammatory process caused by food allergy triggers
2. Downregulate NGF
3. Improve the symptoms of AD.
Methods
Study design
In this randomized, double-blind, placebo-controlled, parallel-group trial, participants diagnosed with AD and food allergies aged 1-8 years were enrolled. The study was conducted at 2 outpatient clinics in the Denver, Colorado, metropolitan area between February 2008 and March 2009. Individual subject participation lasted for approximately 9 weeks. The study was IRB-approved with informed consent and registered with ClinicalTrials.gov. (Quorum Review IRB # 23389/1; ClinicalTrials.gov Identifier: NCT00557284).
Subjects
Thirty-three subjects were screened for the trial; 20 subjects met all inclusion and exclusion criteria. Nine subjects were randomized to receive montelukast and 11 subjects were randomized to receive placebo. Eleven males and 9 females were enrolled. The mean age of participants was 5.4 years.
All entry criteria were assessed at the baseline visit. Using age at the time of screening (1-8 years inclusive), participants with mild to moderate AD affecting at least 5% of the body surface with a total severity score, rated by the study doctor, of 2 for any of the 4 signs, were enrolled. GI symptoms were rated by the Gastrointestinal Symptom Rating Scale (GSRS) and a total score of 2 was necessary for inclusion. If participants had previously tested positive for food allergens by skin or radioallergosorbent test (RAST) test, the previous results were used. If participants had not been tested for food allergens, then the necessary tests (RAST and skin tests) were performed to determine eligibility. Subjects were seen in clinic by a study doctor a total of 4 times, once every 21 to 23 days, over a 9-week study period. Standard of care in the clinic is to avoid foods that may lead to anaphylaxis as determined by RAST or skin testing.
Skin evaluations were performed by the study doctor at study visits using Investigators’ Global Assessment (IGA) rating, severity scores, and percent of body area affected. Blood was taken at visit 1 and 4 and urine samples were taken at each visit. Caregivers filled out weekly assessments of disease control, pruritus evaluation, and AD flare-up cards. GI symptoms were evaluated weekly by the caregiver using the GSRS scale. Visit 1 scores were considered baseline. Non-serious adverse events (NSAES) and serious adverse events (SAES) were recorded, monitored, and reported according to protocol.
The study consisted of 2 treatments arms: those who received montelukast and those who received placebo. All study drugs, montelukast or placebo, were dosed orally one time per day. Subjects aged 2 to 5 years of age received 4-mg chewable tablets and subjects 6 to 8 years of age received 5-mg chewable tablets. Second line therapy provided for treatment of AD flares when symptoms became unacceptable to the participants consisted of 1% hydrocortisone cream applied to affected areas and cetirizine HCl (Zyrtec®) liquid. Subjects 1 to 4 years of age received ½ teaspoon of cetrizine once daily as needed and subjects 5 to 8 years of age received 1 teaspoon once daily as needed.
Evaluations
Demographic information, medical history, and medication history were collected at the initial visit. All concomitant medications were recorded by the caregiver in the diary card and reviewed at study visits for the duration of the trial. Several validated scores were used for evaluation during the trial. The IGA score is a 6-point measure of disease severity based on the clinicians overall assessment of skin lesions: 0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe, and 5=very severe. Subjects did not need to have a specific IGA score to qualify for the trial. The IGA was assessed at each of the 4 study visits. The score recorded at visit 1 was considered the baseline score; caregiver’s assessment score was the evaluation made by the caregiver on the perceived level of disease control over the previous 7 days. Level of control was ranked on a 4-point scale ranging from complete control (0) to uncontrolled disease (3). The caregiver recorded this score on a weekly basis in the diary card. Pruritus (“itch”) assessments were recorded by the caregiver for the previous 24 hours using a 4-point scale, ranging from none (0) to severe (3). This assessment was recorded daily in the diary card. AD flare-ups were documented. A flare-up is defined as a worsening of disease that is unacceptable to the participants and leads to second-line topical steroid use and/or liquid antihistamine use. The caregivers documented flare-ups by recording the use of second-line therapy in the diary card. GI symptoms were recorded weekly on a GSRS-validated scale adjusted for pediatric patients.
Laboratory assessments
Urine samples were obtained at all study day 4 visits for all subjects. The urine sample at visit 1 was considered the baseline. Urine samples were analyzed for levels of Cys-LTs.
Serum samples were collected at the first study visit and the last. A chemistry panel, including albumin, alkaline phosphatase, serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and blood urea nitrogen (BUN) as well as a complete blood count (CBC), were performed as a safety precaution. No safety concerns were identified by these tests. Serum markers were also obtained for immunoglobulin E (IgE), interleukin-5 (IL-5), interleukin-13 (IL-13), tumor necrosis factor-alpha (TNF-α), and NGF. Chemistry and IgE levels were run by Quest Diagnostics. IL-5, IL-13, TNF-α, NGF, and urine LTE-4 were measured using ELISA at ELISATech.
Statistical analysis
Treatment effects were analyzed using repeated measures, including ANOVA methods, which take into account correlation within subjects. Individual contrasts were calculated to compare treatment means of interest. All p-values were two-sided; all analyses were performed using SAS (SAS Institute, Inc., version 9.1).
Results
Primary endpoint evaluations – Skin evaluations
The primary endpoints for skin evaluations were IGA, caregivers’ assessment of disease control (PADC), pruritis assessment, and AD flare-up. Pruritis scores and PADC were measured on a daily basis. AD flare-up was documented by tracking the number of times rescue medicines (liquid cetirizine and/or 1% hydrocortisone cream) were required to control symptoms.
The overall skin level of discomfort using PADC, pruritis, and AD flare-up was greatly improved in subjects using montelukast compared to those using placebo (Tables 1-3). Figure 1 depicts the average weekly pruritus scores by treatment group. Study visits compared to baseline followed a downward trend in the montelukast group, with the difference being most significant by the last visit. When comparing pruritis baseline scores to last study visit scores, the difference between placebo and montelukast was significant (p=0.002). There was a difference in use of cetirizine (p=0.056) and hydrocortisone cream (p=0.054) between the 2 groups, but it was not significant. A similar trend was also noticed for PADC (p=0.248).
-

Table 2:
Mean Change (SD) in Pruritus: Montelukast and Placebo.
-

Table 3:
Mean Change (SD) in Flare-ups: Montelukast and Placebo.
-
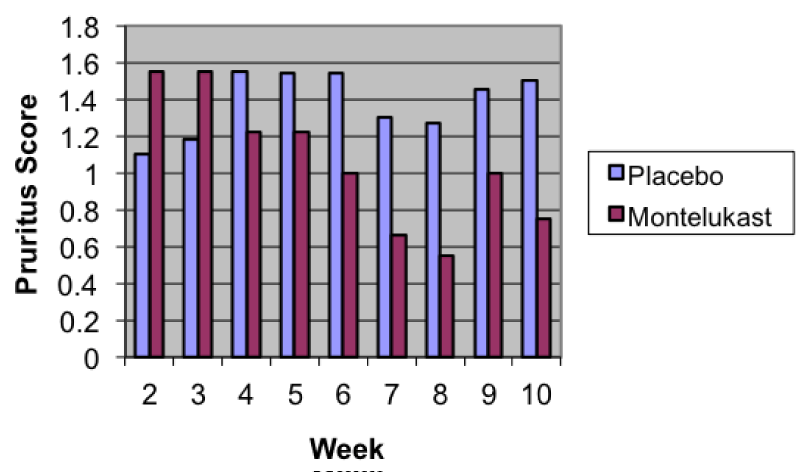
Figure 1:
Average Weekly Pruritus Scores by Treatment.
IGA appeared to be a timed response, with maximum effect noted by visit 4. The severity scoring showed the same trend (Table 4).
-

Table 4:
Mean Change (SD) in IGA Score and Severity Score: Montelukast and Placebo.
Secondary endpoints – Laboratory assessment and GI evaluations
Urinary marker LTE4 was collected at each visit and was reduced in the montelukast group compared to the placebo group (Figure 2).
-
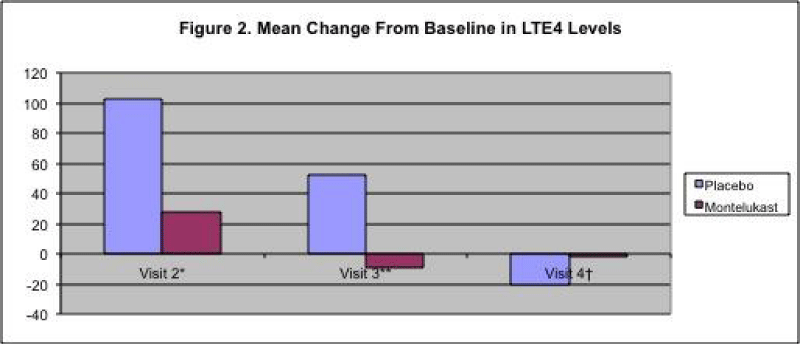
Figure 2:
Mean Change from Baseline in LTE4 Levels.
Laboratory serum markers were collected at baseline and at the last study visit. There were no differences between the study arms, with all levels within normal range limits. Serum markers for TNF-\ alpha and NGF showed a slight difference between the 2 arms (values were calculated using log transformation) (Table 5).
-

Table 5:
Mean Change (SD) in Serum Markers for TNF-α and NGF: Montelukast and Placebo.
IL-13 showed a reduction from baseline. IL-5 levels were all below the sensitivity range of the assay. GI assessments using GSRS scoring were filled out weekly. These data showed a slight difference between arms, with montelukast showing some improvement over time (Table 6).
-

Table 6:
Mean Change (SD) in GRGS Score: Montelukast and Placebo.
Discussion
There are various immune players in the cascade of AD, including T-cells, eosinophils, and mast cells. Mast cells were previously recognized primarily for their granulation upon IgE receptor aggregation in allergic diseases [24-26]. More recently, mast cells have been shown to respond to various infectious pathogens. They can also contribute to chronic inflammatory skin disease or food allergies. For example, a recent study demonstrated that a food protein gliadin could have a potent immunomodulatory effect in atopic persons [27]. Gliadin can be an agonist of IL-8, mediated by CXCR3 engagement.
Contributing factors to eczema include genetic predisposition, a defective skin barrier, and environmental triggers such as microorganisms and allergies. Moreover, mast cells from atopic persons tend to release more inflammatory cytokines following stimulation. The focus of this study was to evaluate the effect of an LTE4 blocker on food allergens that lead to this condition.
The LTE4 pathway is involved in various inflammatory pathways, some mediated by mast cells and other various players. In this study we tested the ability of montelukast to downregulate the inflammatory process caused by food allergy triggers and improve the symptoms of AD.
AD is an inflammatory skin disease that is the end result of various triggers like food allergies, infection, stress and environmental factors. In recent years, increasing evidence has suggested that regulatory T-cells are involved in various skin diseases and play a pivotal role in the etiology of AD. The role of T cells in the development of AD is complex and may involve various mechanisms [6]. Immune abnormalities observed in AD include primary defects, such as epithelial barrier defects and defects in signaling or expression of innate receptors. Others are thought to be secondary to the effects of the adaptive immune response. These include deficiencies in antimicrobial peptides due to the overexpression of T helper 2 cytokines such as interleukin-4 (IL-4) and IL-13. How these components interact is not fully understood [28].
We recently studied the role of perforin and granzyme B as factors that may lead to AD via apoptosis failure. The rationale underlying this study was that recent data suggest that AD is an immunological process resulting from the inability of T-cells or cutaneous tissue to be normally regulated due to a failure in the signaling of apoptosis. As a consequence of this failure, the immunological barrier is lost, which may have later immunological consequences.
The LT4 pathway is involved in various inflammatory pathways mediated by mast cells and many other players. We have studied the role of NGF as a crosstalk between mast cells and the immune system. We have shown that NGF release is upregulated in persons with AD and we speculate that it may play a role in AD.
Our results suggest that montelukast can downregulate the inflammatory pathway initiated by food allergies and leading to AD. We have also shown that montelukast has a signficiant effect on pruritus in patients with AD and food allergies, and we showed a trend in the downregulation of NGF. Although our study was small, we believe that further study is needed to determine if montelukast has a role in early intervention in the allergic march, which begins with eczema, progresses to allergic rhinitis and finally to asthma.
Conclusion
In this randomized, double-blind, placebo-controlled, parallel-group trial in children with food allergies and atopic dermatitis, montelukast led to a reduction in pruritus when compared to placebo, with trends toward a reduction in the use of rescue medication and a reduction in the levels of nerve growth factor.
Acknowledgement
The authors would like to thank Irene Durham, MS, CMPP, for editorial assistance.
Source of Funding
Merck & Co., Inc.
-
-
- Leung DY (2000) Atopic dermatitis. Allergy Immunol 105: 860-876. Link: https://goo.gl/BAOrVX
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA (2004) New insights into atopic dermatitis. J Clin Invest113: 651-657. Link: https://goo.gl/kT5OoQ
- Leung DY, Bieber T (2003) Atopic dermatitis. Lancet 361: 151-160. Link: https://goo.gl/Ob8heJ
- Guido K, Zitvogel L (2007) Death, danger, and immunity: an infernal trio. Immunol Rev 220: 5-7. Link: https://goo.gl/HvReQA
- Verhagen J, Akdis M, Traidl-Hoffman T, Schmid-Grendelmeier P, Hijnen D, Knol EF, et al. (2006) Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol117: 176-183. Link: https://goo.gl/9QoiRy
- Ogg G (2009) Role of T-cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy 39: 310-316. Link: https://goo.gl/xN8UKf
- Chatila TA (2008) Molecular mechanisms of regulatory T-cell development. In: Blazer K, ed. T-cell Regulation in Allergy, Asthma and Atopic Skin Diseases, 1st ed. Switzerland. Chem Immunol Allergy 94: 16-28. Link: https://goo.gl/qrZZGv
- Adamek-Guzik T, Guzik TJ, Czerniawska-Mysik G, Korpanty G, Mastalerz L, et al. (2002) Urinary leukotriene levels are increased during exacerbation of atopic eczema/dermatitis syndrome: relation to clinical status. Allergy 57: 732-736. Link: https://goo.gl/HDV7Bd
- Worm M, Vieth W, Ehlers I, Sterry W, Zuberbier T (2001) Increased leukotriene production by food additives in patients with atopic dermatitis and proven food intolerance. Clin Exp Allergy31: 265-273. Link: https://goo.gl/95iYje
- Khan S, Orenstein SR (2002) Eosinophilic gastroenteritis: epidemiology, diagnosis and management. Paediatric Drug 4: 563-570. Link: https://goo.gl/4dXLJT
- Daneshjoo R, Talley N (2002) Eosinophilic gastroenteritis. Curr Gastroenterol Rep 4: 366-372. Link: https://goo.gl/XKy6YP
- Worm M, Vieth W, Ehlers I, Sterry W, Zuberbier T (2001) Increased leukotriene production by food additives in patients with atopic dermatitis and proven food intolerance. Clin Exp Allergy 31: 265-273. Link: https://goo.gl/W6IqX0
- Hakugawa J, Bae S J, Vinesa M A, Tanaka Y, Katayama I (1997) The inhibitory effect of LTB4 antagonist on eosinophil infiltration in cutaneous and gut late phase response in BALB/c mice sensitized with ovalubumin (OVA). Arerugi-Japanese J Allergol 46: 42-48. Link: https://goo.gl/XQhD0D
- Melamed I, Franklin RA, Brodie C, et al. (1995) Signaling of B-lymphocytes by nerve growth factor: tyrosine phosphorylation of phospholipase Cg1, MAP2-Kinase and gp140trk. J Immunol 154: 4965-4972.
- Franklin RA, Brodie C, Melamed I, et al. (1993) Nerve growth factor induced activation of MAP2-Kinase and P90rsk in B-lymphocytes. J Immunol 150: 181A.
- Melamed I, Turner C, Gelfand EW (1994) Nerve growth factor triggers microfilament assembly and paxillin phosphorylation in B-lymphocytes. J Clin Immunol 93: 285. Link: https://goo.gl/BBhEkw
- Melamed I, Turner CE, Aktories K, Kaplan D, Gelfand EW (1995) Nerve growth factor (NGF) triggers microfilament assembly and paxillin phosphorylation in B-lymphocytes. J Exp Med 1995; 181: 1071-1079. Link: https://goo.gl/l2ZFd2
- Franklin RA, Brodie C, Melamed I, Terada N, Lucas JJ, et al. (1995) Nerve growth factor induces activation of MAP2-kinase and p90rsk in human B-lymphocytes. J Immunol1995; 154:4965-72. Link: https://goo.gl/f1Lx9Q
- Melamed I, Kelleher CA, Franklin RA, et al. (1993) Signaling of B-lymphocytes by nerve growth factor: gp140trk as a neuro-immune adaptor. Molec Biol Cell 4: 123.
- Nockher WA, Renz H (2003) Neurotrophins in inflammatory lung disease; modulators of cell differentiation and neuro-immune interactions. Cytokine Growth Factor Rev 14: 559-578. https://goo.gl/jGqjLz
- Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, et al. (2002) Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol 147: 71-79. Link: https://goo.gl/JFL6Uw
- La Sala A, Corinti N, Federici M, Saragovi HU, Girolomoni G (2000) Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J Leukoc Biol68: 104-110. Link: https://goo.gl/a0BCdn
- Wang GI, Hseih WS, Guo YL (2008) Neuro-mediators as predictors of pediatrics atopic dermatitis. Clin Exp Allergy 38: 1302-1308. Link: https://goo.gl/eehcbb
- Bresciani M, Laliberte F, Laliberte MF, Gramiccioni C, Bonini S (2009) Nerve growth factor localization in the nasal mucosa of patients with persistent allergic rhinitis. Allergy64: 112-117. Link: https://goo.gl/73mWL2
- Escae D, de Benedetto A, Beck LA (2004) Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep4: 276-284. Link: https://goo.gl/AqgiWe
- Alenius H, Laounini D, Woodward A, Mizoguchi E, Bhan AK, et al. (2002) Mast cells regulate IFN gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J Allergy Clin Immunol 109: 106-113. Link: https://goo.gl/gpPXMV
- Varjonen E, Vainio E, Kalimo K (2000) Antigliadin IgE--indicator of wheat allergy in atopic dermatitis. Allergy 55: 386-391. Link: https://goo.gl/4Y7MTo
- Niebuhr M, Werfel T (2010) Innate immunity, allergy and atopic dermatitis. Curr Opin Allergy Clin Immunol 10: 463-468. Link: https://goo.gl/AuuFMX










Table 1:
Mean Change (SD) in PADC: Montelukast and Placebo.
Placebo
Montelukast
P value
Visit 2 – 1
-.30(.67)
-.57(.73)
0.4398
Visit 3 – 1
-.33(.50)
-.44(.88)
0.7477
Visit 4 – 1
-.10(1.4)
-.78(1.1)
0.2479