Author(s):
Moussa Guèye1*, Justin Kantoussan2 and Mbaye Tine2
1IRD, route des hydrocarbures, BP 1386, 18524 Dakar, Sénégal
2UFR des Sciences Agronomiques de l’Aquaculture et des Technologies Alimentaires (UFR S2ATA), Université Gaston Berger (UGB), Route de Ngalléle BP 234, Saint-Louis, Sénégal
Received: 30 August, 2016; Accepted: 19 September, 2016; Published: 20 September, 2016
Moussa Guèye, IRD, route des hydrocarbures, BP 1386, 18524 Dakar, Sénégal, E-mail:
Guèye M, Kantoussan J, Tine M (2016) Common Garden Experiments Confirm the Impact of Salinity on Reproductive Traits that is Observed in Wild Populations of the Back-Chinned Tilapia Sarotherodon melanotheron. Int J Aquac Fish Sci 2(1): 031-037. DOI: 10.17352/2455-8400.000017
© 2016 Guèye M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Experiment; Impact; Reproduction; Salinity; Tilapia
The black-chinned tilapia Sarotherodon melanotheron is a very hardy species, particularly notable for its ability to tolerate a wide range of environmental salinities. The impact of environmental salinity on reproductive traits has been well documented in this species under natural conditions, but few studies have been experimentally conducted to prove such a relationship. This study assessed the effects of different salinities on the reproductive traits of S. melanotheron in experimental conditions. The number of spawning eggs as well as the mean number and frequency of incubation showed significant relationships with ambient salinity. The average number of incubations and spawning eggs were lower in hypersaline water (70 psu) compared the freshwater (0 psu) and seawater (35 psu). However the egg quality, as measured by the percentage of black eggs, was higher in hypersaline water. These variations in reproductive traits do not reflect genetic differences among individuals because fish used in common garden experiments were obtained by natural breeding from couples, the initial brood stock of which was from the same wild population. The results therefore confirm findings of previous studies in natural habitats, where variations in salinity are associated with differences in reproductive traits among populations. The lower number and frequent incubations recorded in hypersaline water could be an adaptive response to sustain the population dynamics despite the constraints imposed by extreme salinity.
Introduction
The increasing demand for food resources due to growing human population has stimulated the intensification of tilapia farming in recent years, especially in Asian and South American countries such as Taiwan, Thailand, Philippines, Indonesia, Brazil and more recently in southern zones of United States, particularly in Florida and Texas [1]. In Africa, tilapia were intensively cultured only in Egypt and Sudan until recently but, with the collapse of wild stocks, the farming of tilapines is growing rapidly in many countries [2]. Tilapias have become the third most important species group of farmed fish in the world after carp and salmonids with a global production of approximately 3,200,000 metric tons in 2010 [3]. The worldwide economic importance of this cichlid family is therefore growing rapidly, especially in countries where they represent an important source of dietary protein. This has led to increased research efforts for the improvement of aquaculture production, with focus on the identification of ideal environmental conditions for optimal growth and reproductive performance, best strain selection and sex control for fast growth and maximum productivity, and better feed conversion ratio [4-6]. For years, the intensive farming of tilapia for large-scale commercial production was almost exclusively limited to three species of the genus Oreochromis (O. niloticus, O. aureus and O. mosambicus) due to their best growth potential compared to the species of the genus Sarotherodon cultured in semi-commercial production systems essentially for local domestic consumption [7,8]. The growth rates obtained in the genus Sarotherodon are not impressive for commercial culture. However, the species has other potential such as fast growth, high adaptability and a wide dietary spectrum that promote its culture, which is now spreading to many countries of the world [9].
Although the growth potential of fish in aquaculture depends on the intrinsic growth potential of the species itself, environmental factors play also an important role. The black-chinned tilapia Sarotherodon melanotheron is known for its ability to tolerate a wide range of environmental factors including salinity. The species is one of the rare tilapia that can live in waters with very high salinities, up to 130 psu [10,11]. It is also probably the only tilapia that is able to reproduce in such high salinities although, at certain levels, salinity has marked influences on reproductive characteristics. Several comparative studies on wild populations have shown differences in reproductive traits such as age and size at maturity, and in fecundity, as a function of salinity, particularly in Senegalese and Gambian ecosystems where salinity shows considerable spatiotemporal variations [11,12].
These studies indicate that variations in salinity are the ultimate cause of these differences in reproductive traits, because they are the most dominant environmental factor, predominating over all other abiotic stressors. Nonetheless, this has not been rigorously tested. Many biotic/abiotic factors, such temperature, precipitation, competition, food availability and efficiency at foraging, could also directly or indirectly contribute to these differences. Furthermore, although unlikely, it is also conceivable that these differences also reflect genetic adaptation to local conditions. The species has strong population genetic structure, which is assumed to be linked to low larval dispersal ability due to their mouth-brooding reproductive behavior [13-15]. Genetic differentiation has been observed among populations even on a microgeographic scale in the Ebrié lagoon [16]. It has also been found that samples collected at the same location at different times had more genetic differentiation than those collected at different locations of the lagoon, suggesting that the genetic structure of S. melanotheron populations is more dependent upon the degree of relatedness between individuals of the same generation than on geographical distance [16].
Common garden experiments are commonly employed to distinguish environmental from genetic influences on phenotypic variations among populations. These approaches allow separation of the genetic and environmental contributions to phenotypic variations and have been successfully used in a range of taxa including fishes [17-19]. When conducted with offspring from a single population with the same genetic background, common garden experiments can also be used to reveal which, among a group of environmental factors, is responsible for inter-individual variation in a given phenotypic trait in a single population [20]. This is achieved by varying one environmental parameter while keeping the others constant.
In this study, we used common garden experiments to investigate the influence of ambient salinity on reproductive traits in S. melanotheron. The main aim was to confirm that differences in reproductive characteristics in wild populations in Senegalese and Gambian estuaries are related to spatiotemporal variations in salinity. Our approach also will give some indications on salinities for optimal reproductive and growth performance, which is crucial for farming of the species. Fish used in the experiments were from a single population maintained under laboratory conditions for several generations. Fish were exposed to different salinities (freshwater: 0 psu, seawater: 35 psu and hypersaline water: 70 psu) while other environmental factors and feeding conditions were held constant. More specifically, we assessed whether exposition to these salinity conditions induce differences in spawning number and frequency, the number, size and quality of spawned eggs, and the number of incubations as well as relation of these variables with body size.
Material and Methods
Experimental approach
Experiments were performed on adults maintained at the GAMET (Groupe Aquaculture Méditerranéenne Et Tropicale) experimental facility in Montpellier, France. Fish were obtained by natural breeding and came from couples whose initial broodstock was collected from the Niayes region (Dakar, Senegal) and maintained at GAMET since 1998. Fish were reared for 16 weeks in six tanks of 430 liter each, two for each salinity condition (freshwater: 0 psu, seawater: 35 psu and hypersaline water: 70 psu). Young adults were reared in parallel in extra hypersaline water tanks to assess the impact of individual size on salinity tolerance and reproductive characteristics. Each tank was equipped with a thermostat to maintain a constant temperature of 27°C. The tanks functioned within a recirculated thermo regulated water system and each of them was equipped with a system of mechanical and biological filtration that maintained water quality high. Water was aerated vigorously to ensure optimal oxygen saturation. All tanks were initially filled with freshwater from a borehole and then salt was progressively added until the desired salinity (35 or 70 psu) was reached. The commercial salt mixture contained all the components of seawater and was initially dissolved in a bucket with water from the system before being diluted into the tank. For the hypersaline tanks, the salinity was increased by 2g of salt per liter of water every day until 70 psu. The rise in salinity was, however, stopped for two weeks after it reached 56 psu, due to high mortalities. The effective duration of the experimental follow-up phase was ten weeks and the phase of rise in salinity from 35 to 70 psu (considered an acclimation phase) took six weeks. Water was partially changed (about 1/3 of volume) at regular intervals to maintain quality high. Portions of PVC pipes were placed at the bottom of the tank to provide shelter and reduce aggressiveness between individuals in this territorial fish.
Tank stocking and physicochemical parameters
Before stocking the tanks, fish were anesthetized in a bath containing 0.3 ml/l phenoxy-2-ethanol, measured, weighed and marked individually with an electronic mark (PIT tag). Each tank was filled with ten fish comprised of five females and five males of equivalent size. Fish were fed with commercial processed fish feed containing 40% crude proteins and distributed six days a week for a fixed ration equivalent to 0.5-1% of the biomass. All individuals were monitored weekly to collect eggs in the male mouth brooders.
Physicochemical parameters (salinity, temperature, dissolved oxygen, ammonia and nitrite) were monitored regularly. Salinity was measured and adjusted once or twice per week using an optical refractometer. The temperature of the tanks was set at 27°C and maintained constant during the whole experimentation period. The concentration of dissolved oxygen was maintained around the saturation. These two parameters were monitored once or twice every week with an oximeter probe used for simultaneous measurements of water temperature and dissolved oxygen. The rate of nitrite and ammonium were controlled once or twice a week depending on the state of pollution in the tanks. Control of nitrate and nitrite was performed using a commercial kit used for simultaneous measurements of nitrite and ammonium concentrations.
Fish monitoring and control of incubation
The fish were monitored at the beginning of each week. During each test, the male incubators were identified by morphological and ethological criteria such as swelling of the lower part of the oral cavity, lack of aggressiveness, reduced feeding activity and mobility. Incubator males were captured with a net, and then forced to release the offspring in a water bucket by gently opening the mouth. The eggs were immediately recovered and stored in tubes containing water from the same tank.
Treatment of collected eggs
After collection, eggs were maintained alive in gently aerated water at their appropriate salinity. Developmental stages of the live eggs were immediately determined under an optical microscope, based on the Shaw and Aronson [21] scale in Tilapia macrocephala (synonym of the S. melanotheron). The diameter of the eggs was determined by image analysis with the Image J software. The eggs were then counted manually, and weighed to determine their total mass. A sample of 100 eggs was removed, weighed and the volume determined for the determination of egg density. Another sample of 100 eggs was weighed and dried at 105°C for 24 hours to determine water content. After drying, the sample was burnt at 550°C for 6 hours to determine ash content.
Fish condition
The condition factor (K), the most widely used morphometric index, provides information on the physiological state of the fish. Indeed, fish individuals that live in constrained environments generally have low body weight and size compared to those inhabiting favorable environments. The condition factor was compared between and within rearing environments at the beginning and the end of experience. Condition factor was calculated using the standard formula:
K = (P / LF3 ) * 105
where P is the total individual weight and the LF the fork length .
Reproductive rate
The number of incubations as a function of the number of females or males in the tanks was used as an index of reproductive rate in each rearing environment. This reproduction rate is indicated by the percentage of males incubators observed during successive samplings conducted in the course of the experimentation. This percentage also gave an idea of the number of females that were sexually active.
Statistical analysis
The U-test was used to compare the means of the salinity, temperature and O2 between 9h and 16h in each rearing environment. This was done to compare the variation of the daily experimental conditions between morning and afternoon during all experimental period. The variations of the salinity, temperature, O2, NH4 and NO2 between freshwater, seawater and hypersaline water were compared by using one-way ANOVA test. The same test was used to compare fish condition, oocyte weight and oocyte density between the three salinity conditions. To compare the number of incubations, number of spawning eggs, water and ash content in eggs between different water salinities, we used Kruskal-Wallis rank sum test. A linear regression was applied to test the relationship between the number of incubated eggs and the weight of male incubators. For all tests, a probability less than 5% was used as fiducial level of significance. All statistics were performed with the R statistical environment [22].
Results
Physicochemical factors
Table 1 shows that in each tank, the daily experimental conditions (between morning and afternoon) were comparable throughout the experimentation period. As previously mentioned, experimental tanks were initially filled with freshwater and then salt was progressively added until the desired salinity (35 or 70 psu) was reached. The differences in salinity between morning (9h) and afternoon (16h) were recorded during the phase of increase in salinity. The maximum daily salinity variation in SW and HW tanks during the increase in salinity were not significant (31.80±5.74 vs 33.06±0.51 for SW) and (35.92±12.31 vs 36.03±10.77 for HW) (Table 1). There were no significant changes in salinity, water temperature and dissolved oxygen between morning and evening (U-test, p>0.05). The comparison between rearing environments showed no significant differences in water temperature and dissolved oxygen (Table 2). The variation in ambient salinity between rearing environments was significant (Table 2). The levels of NH4 and NO2 were significantly different between rearing environments with higher values in seawater and hypersaline water tanks in comparison to freshwater tanks (ANOVA, p<0.05). Increased concentrations of nitrite were observed in seawater and hypersaline water tanks in the early phases of the experimentation, which were probably related to the fact that nitrifying bacterial flora was slower to develop in these environments.
-

Table 2:
Variation in physicochemical parameters between rearing environments (ANOVA-test: ns = no significant; ** significant at probability level 0.01; *** significant at probability level 0.001).
-

Table 3:
Comparison of fish condition factor within rearing environment at the beginning and the end of experience.
Mortality rate in the rearing environments
The highest mortality rates were recorded in seawater (10%) and hypersaline water (15%) tanks while, in the freshwater tanks, mortality rate was 5%. The mortality rates were higher at the beginning of the experiment during the phase of rise in salinity from 15 to 35 psu for the seawater tanks and from 15 to 70 psu for hypersaline water tanks. Frequent mortalities were observed in the hypersaline thanks when the salinity reached 55 psu. The rise in salinity was therefore stopped for few days to allow fish to acclimate before continuing the experiment. In the three rearing environments, the highest mortality rates were recorded in females (40%) compared to males (20%). The aggressiveness of males, that can kill females, seemed to increase with salinity.
Condition factor
There were no significant differences in condition factor between individuals in the different tanks at the beginning of the experiment (ANOVA, p>0.05; Figure 1a) whereas at the end of the experiments, the average condition factor was higher in hypersaline water compared to the other rearing environments (freshwater and hypersaline water), with no significant difference between these latter conditions (ANOVA, p<0.05; Figure 1b). Condition factor decreased significantly with time in both freshwater and seawater tanks, but not in the hypersaline tanks (Table 3).
-
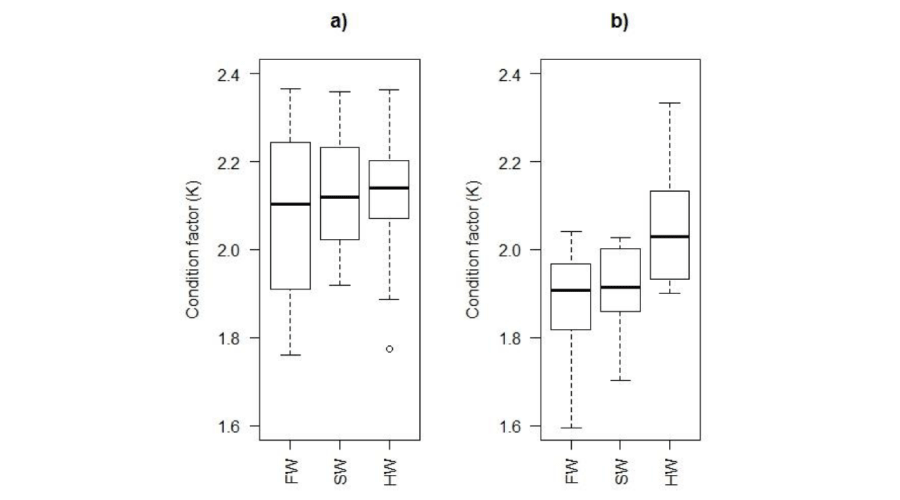
Figure 1:
Comparison of fish condition factor between rearing environments at the beginning (a) and the end (b) of experience. FW: Freshwater; SW: Seawater; HW: Hypersaline water.
Number of incubation
The total number of incubations was significantly lower in hypersaline tanks in comparison to the other conditions (Figure 2). The average number of incubations per week was significantly lower in hypersaline tanks than in freshwater or seawater tanks (Kruskal Wallis test, p < 0.05), with no significant difference between these latter two rearing conditions. Fish in freshwater and seawater incubated once a week whereas those in hypersaline tanks incubated once every two weeks (Figure 3). The number of incubations was inversely correlated with body mass in males (r = 0.64; p<0.05) (Figure 4).
-
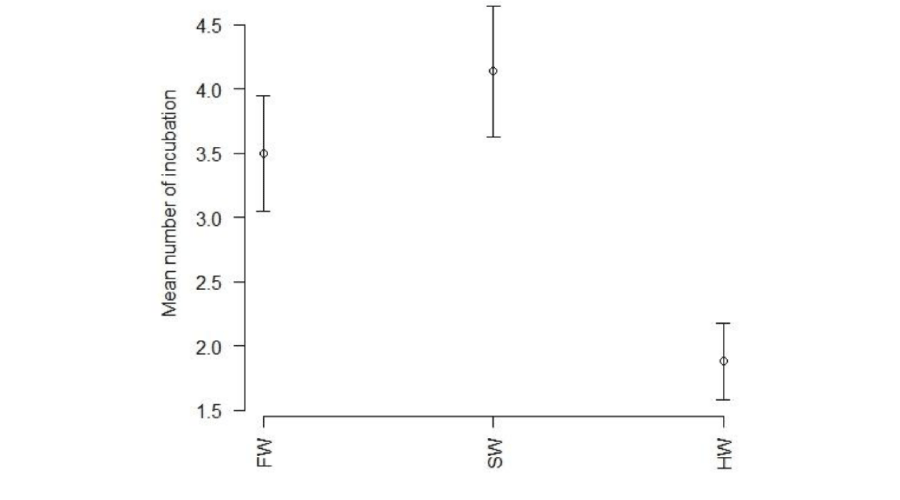
Figure 2:
Comparison of the average number of incubation (Kruskal-Wallis test, p<0.001). FW: Freshwater; SW: Seawater; HW: Hypersaline water.
-
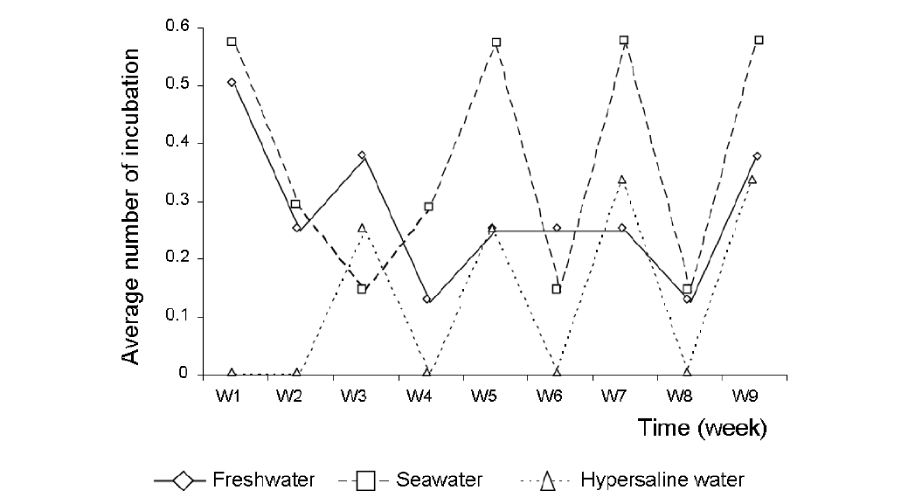
Figure 3:
Temporal evolution of the average number of incubations in fish groups reared in different salinity conditions. W1 = week 1, W2 = week 2, W3 = week 3, W4 = week 4, W5 = week 5, W6 = week 6, W7 = week 7, W8 = week 8, W9 = week 9.
-
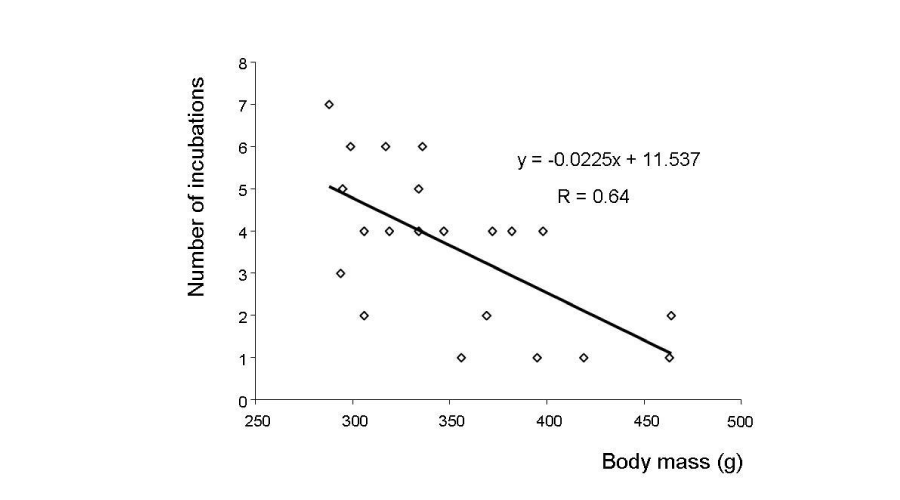
Figure 4:
Linear regression between male body weight and the average number of incubation recorded during the experiment. g = gram.
The number of incubation per female was 0.30±0.06, 0.33±0.13 and 0.16±0.10 in freshwater, seawater and hypersaline water, respectively. In males, it was 0.30±0.06, 0.26±0.098 and 0.08±0.06 in freshwater, seawater and hypersaline water, respectively.
Number of incubated eggs, blank eggs and incubation mass
The average number of eggs incubated by males was significantly higher in freshwater and seawater than in hypersaline water (Figure 5a). No significant relationship was found between the number of incubated eggs and male body mass in freshwater (r2 = 0.33; p = 0.23), seawater (r2 = 0.35; p = 0.15) or hypersaline water (r2 = 0.22; p = 0.22).
-
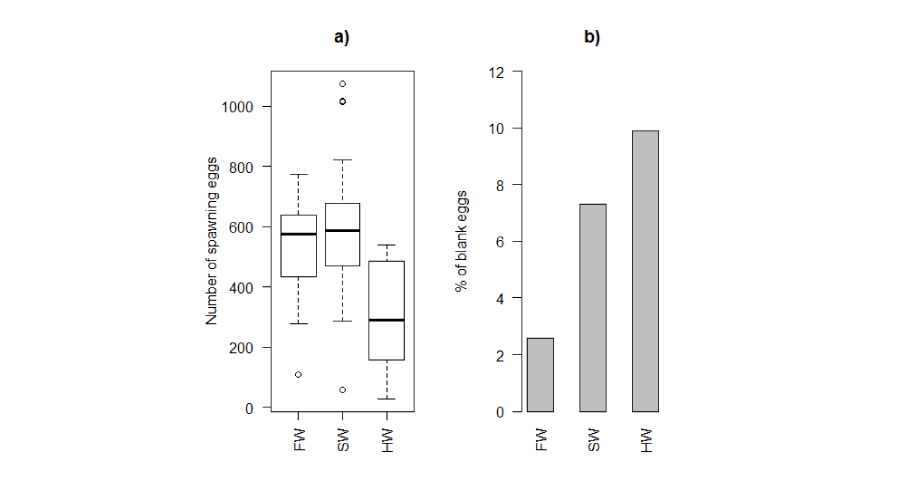
Figure 5:
Number of spawning (a) and blank (b) eggs in different rearing environments (Kruskal-Wallis chi-squared = 9.0479, p-value = 0.01085). FW: Freshwater; SW: Seawater; HW: Hypersaline water.
The percentage of blank eggs was higher in hypersaline tanks in comparison to seawater and freshwater (ANOVA test, p<0.05) (Figure 5b). There was no significant difference in the percentages of blank eggs between seawater and freshwater.
The average total mass of eggs incubated by males was significantly higher in freshwater and lower in hypersaline water. There was no relationship between the number of incubated eggs and male body mass in freshwater (r2 = 0.01; p = 0.99), seawater (r2 = 0.10; p = 0.68) or hypersaline water (r2 = 0.37; p = 0.62).
Eggs diameter, weight and density
No significant difference in diameter was observed for eggs of same developmental stage (ANOVA test, p>0.05). Egg mass showed no significant difference between salinity conditions for any stage of development (Figure 6a). Likewise, egg density did not show significant differences among rearing environments for any stage of development (ANOVA test, p>0.05; Figure 6b). In some cases, the calculated densities were lower than those in the tanks. It should be noted that, in natural environments, all egg densities calculated (unfertilized eggs) are higher than their surrounding water.
-
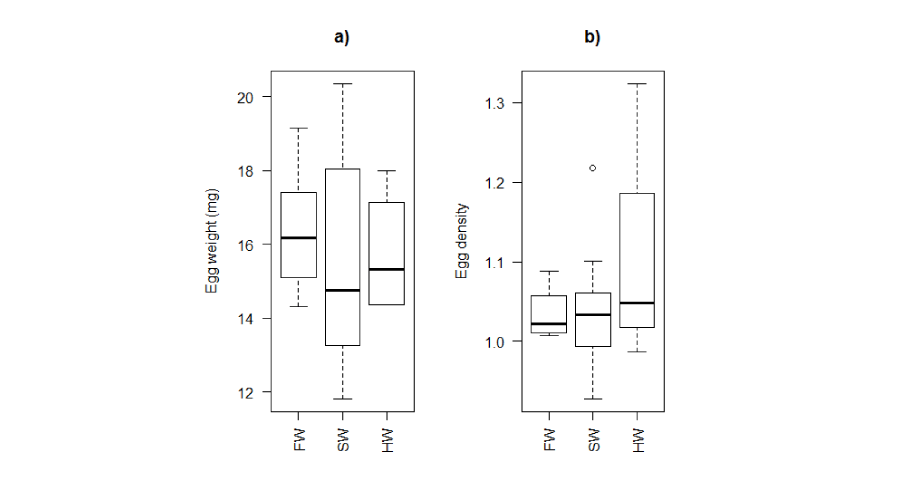
Figure 6:
Eggs weight (a) and densities (b) in different rearing environments. FW: Freshwater; SW: Seawater; HW: Hypersaline water.
Water and ash content
The water content of eggs decreased significantly with the stage of development but for the same stages of development, the water content did show any significant difference between salinities (Figure 7a). The comparison of water content in different developmental stages revealed significant differences between the young stage and all other stages except for the eyed-stage.
No significant difference in ash content was observed between eggs at different stages of development. The comparison of the ash for the same stage of development showed no difference between rearing conditions (Figure 7b). The comparison of the ash content between the different stages of development did not show any significant differences.
-
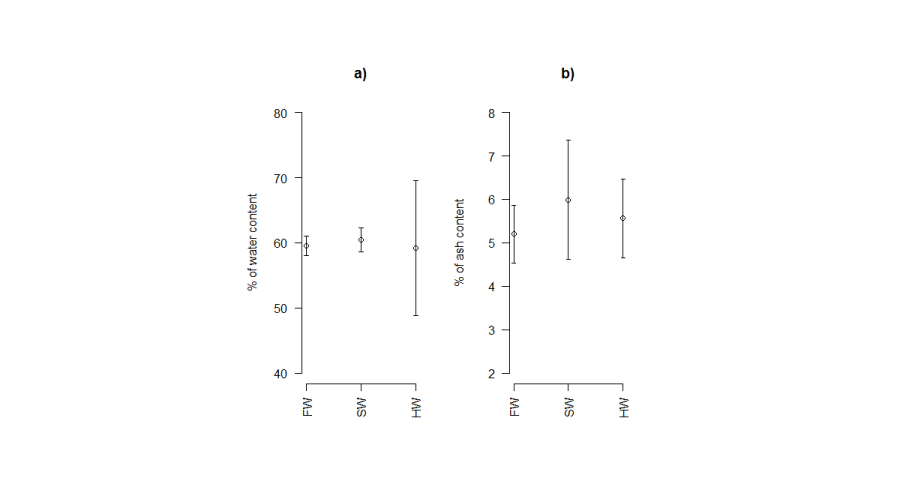
Figure 7:
Percentage of water (a) and ash (b) contents of eggs in different rearing environments. FW: Freshwater; SW: Seawater; HW: Hypersaline water.
Discussion
The results from common garden experiments showed important differences in S. melanotheron reproductive traits among rearing environments. The results are in accordance with those from natural environments, where strong salinity/reproductive traits associations have been found [10,11]. Fish used in this study are from common ancestral parents maintained in laboratory conditions for several generations. It is, therefore, unlike that variations in reproductive traits reflect genetic differences among individuals. Moreover, while the populations where the initial bloodstocks were collected in the Niayes area in Senegal are genetically very structured [23-25], fish used in this study were obtained by natural breeding from couples whose parents were maintained in laboratory conditions for several generations. Also, apart from salinity, all the other physicochemical factors such as temperature, photoperiod, dissolved oxygen and feeding conditions were similar across rearing environments. Therefore, the variations in reproductive traits observed here reflect the differences in salinity among the rearing environments. The main and individual frequency of incubation in hypersaline tanks, which was half (one vs. two weeks) that of the other rearing environments, could be an adaptive strategy to extreme salinities. Indeed, reproductive individuals would decrease the number of larvae produced and, therefore, spend more energy in osmoregulation. Individuals subject to high salt-stress in hypersaline environments would invest more energy in osmoregulation compared to individuals reared in freshwater and seawater that could spend large amounts of their energetic reserves in reproduction. The acclimation to extreme salinities is energetically costly, particularly in hypersaline conditions where individuals have to favor the survival at the expense of reproduction. Such an adaptive strategy has been observed in wild populations inhabiting the hypersaline areas of the Saloum Estuary [11].
The inverse correlation of the number or frequency of incubation with body weight in different rearing environments indicates that the large size is not the only criteria that confers males their dominance. It is a known fact that in fishes including tilapias, young adults produce higher numbers of eggs than old individuals [26]. This can be attributed to the fact that smaller individuals are more resistant to high salt-stress in hypersaline environments and would invest more energy in reproduction compared to large individuals that would spend large amounts of their energetic reserves in osmoregulation. The acclimation to extreme salinities is energetically costly, particularly in large individuals because the osmoregulatory activity is supposed to increase with body surface. This high cost may reduce the amount of energy that would be allocated to reproduction. The salinity 70 psu is considered as the threshold for salt tolerance by S. melanotheron at which the saline stress is high enough to impact biological traits such as growth and reproduction. We have observed that males with higher incubation rates had lower size, which might be explained by higher energy investment on reproduction rather than on growth. This observation is consistent with findings of studies on natural environments that smaller individuals of S. melanotheron inhabiting the hypersaline zones of the Saloum Estuary have a higher reproductive activity and lower growth rates than those living in less salted areas. This interpretation seems to be inconsistent with the absence of significant difference in fish condition in hypersaline tanks, which can be attributed to the fact that some males were not in couple because of the limited number of females. The incubation was, therefore, carried out only by dominant males, which allowed the remaining males to essentially dedicate their energy to osmoregulation and growth. This can also explain the lower condition factor of fish in hypersaline water tanks at the end of the experiments.
The lower number of spawning eggs in hypersaline water as well as the number of eggs incubated in the mouth of each male can be attributed to poor quality of eggs and/or a low fecundation success, as evidenced by the higher percentage of blank eggs in this salinity condition. It could be also that part of spawned eggs was swallowed or spited out by incubator males due to their aggressiveness, which was probably higher in hypersaline tanks. The high percentage of death in hypersaline water tanks during the experimentation (15% vs. 10% and 5% in seawater and freshwater, respectively) strongly suggests an increase of male aggressiveness as the salinity increases [27]. Egg density was reported to be positively correlated with ambient salinity in natural environment, which was interpreted as an adaptive response that allow maintaining eggs at the surface of water whatever the hydrological changes.
It is a common fact that in fish, the egg size is the less reproductive variable parameter [28], which is in accordance with our results in S. melanotheron males that did not show significant relationship between egg number and weight in all rearing environments. These results are, however, not consistent with those reported by Duponchelle and Legendre [29], who have observed an inverse relationship between number and egg weight. The absolute fecundity was not also correlated with females’ size as commonly observed in fish, which may be due to the fact the size range considered in our study was not large enough (genitors with similar size) to induce significant correlations between these two parameters. This absence of correlation can also be explained either by the fact that the total number of eggs produced by females was incubated by different males or males swallowed part of the eggs due to an increased stress when picking up the eggs. This phenomenon of swallowing eggs has been previously observed in S. m. melanotheron by Legendre and Trebaol [30]. Nonetheless, in contrast to our results, these authors found significant correlations between the number of incubated eggs and male size.
Conclusion
Although all differences in reproductive traits observed in wide populations of S. melanotheron cannot be exclusively attributed the variations in ambient salinity, this study provides evidence that hypersaline conditions are stressful for S. melanotheron to an extent that they affect its reproductive performances. It should be, however, mentioned that large changes in reproductive function begin to occur when the salinity was around 70 psu. At this salinity fish fitness was not, however, deeply affected as evidenced by the absence of significant differences in fish condition between rearing environments. While young adults in hypersaline conditions never reproduced during the experiment, their condition factor and probably their growth performances have increased. This suggests that among the vital functions, the reproduction is the most sensitive to ambient salinity in the subspecies S. melanotheron. Although it cannot be concluded that differences in reproductive characteristics of wild populations of S. melanotheron are exclusively due phenotypic plasticity, our results suggest that the salinity is the most predominant environmental factor that affects the reproduction of the species in the field.
Acknowledgements
The authors thank IRD (Institut de Recherche pour le Développement) for financial support. Moussa Guèye benefited from IRD grant for his PhD thesis.
-
-
- FAO (2012) La situation mondiale des pêches et de l’aquaculture 2012. FAO Rome: 261.
- Feidi IH (2010) Tilapia markets in the Middle East and North Africa demand trends and outlook. Proceedings of the 3rd International Technical and Trade Conference and Exposition on Tilapia. Kuala Lumpur, Malaysia.
- Kevin F (2010) Potential to Increase Global Tilapia Production. University of Arizona, United States.
- Kobayashi S, Alimuddin, Morita T, Miwa M, Lu J, et al. (2007) Transgenic Nile tilapia (Oreochromis niloticus) over-expressing growth hormone show reduced ammonia excretion. Aquaculture 270: 427-435.
- Lim C, Yildirim-Aksoy M, Li MH, Welker TL, Klesius PL (2009) Influence of dietary levels of lipid and vitamin E on growth and resistance of Nile tilapia to Streptococcus iniae challenge. Aquaculture 298: 76-82.
- Pongthana N, Nguyen NH, Ponzoni RW (2010) Comparative performance of four red tilapia strains and their crosses in fresh- and saline water environments. Aquaculture 308: S109-S114.
- Gilles S, Amon-Kothias JB, Jean-François A (1998) Comparison of brackish water growth performances of Sarotherodon melanotheron (Cichlidae) from three West African populations. In: Genetics and Aquaculture in Africa (ed. by J.F. Agnese): 199-210. ORSTOM, Paris, France.
- Avarre, J.-C., Guinand, B., Dugué, R., Cosson, J., Legendre, M., Jacques Panfili, J. &Durand, J.-D. (2014) Plasticity of gene expression according to salinity in the testis of broodstock and F1 black-chinned tilapia, Sarotherodon melanotheron heudelotii. PeerJ 2: e702.
- Campbell D (1987) A review of the culture of Sarotherodon melanotheron in West Africa. UNDP/FAO African Regional Aquaculture Centre, Aluu, Port Harcourt, Nigeria. Working Paper ARAC/87/WP/5, 20.
- Panfili J, Mbow A, Durand JD, Diop K, Diouf K, et al. (2004) Influence of salinity on the life-history traits of the West African black-chinned tilapia (Sarotherodon melanotheron): comparison between the Gambia and Saloum estuaries. Aquatic Living Resourses 17: 65-74.
- Guèye M, Kantoussan J, Tine M (2013) The impact of environmental degradation on reproduction of the black-chinned tilapia Sarotherodon melanotheron from various coastal marine, estuarine and freshwater habitats. Comptes Rendus Biologies 336: 342-353.
- Panfili J, Thior D, Ecoutin JM, Ndiaye P, Albaret JJ (2006) Influence of salinity on the size at maturity for fish species reproducing in contrasting West African estuaries. Journal of Fish Biology 69: 95-113.
- Gourène B, Pouyaud L, Agnèse JF (1993) Importance de certaines caractéristiques biologiques dans la structuration génétique des espèces de poissons : le cas de Ethmalosa fimbriata et Sarotherodon melanotheron. J Ivoirien Océano. Limnol. Abidjan 2: 55-69.
- Pouyaud L (1994) Génétique des populations de tilapias d’intérêt aquacole en Afrique de l’Ouest. Relations phylogénétiques et structurations populationnelles. Ph.D. Thesis, University of Montpellier, France.
- Falk TM, Teugels GG, Abban EK, Villwock W, Renwrantz L (2003) Phylogeographic patterns in populations of the blackchinned tilapia complex (Teleostei, Cichlidae) from coastal areas in West Africa: support for the refuge zone theory. Molecular Phylogenetics and Evolution 27: 81-92.
- Pouyaud L, Desmarais E, Chenuil A, Agnèse JF, Bonhomme F (1999) Kin cohesiveness and possible inbreeding in the mouth brooding tilapia Sarotherodon melanotheron (Pisces Cichlidae). Molecular Ecology 8: 803-812.
- Imsland AK, Jonsdottir ODB (2003) Linking population genetics and growth properties of Atlantic cod. Rev Fish Biol Fish 13: 1-26.
- Conover DO, Clarke LM, Munch SB, Wagner GN (2006) Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. Journal of Fish Biology 69: 21-47.
- Hutchings JA1, Swain DP, Rowe S, Eddington JD, Puvanendran V, et al. (2007) Genetic variation in life-history reaction norms in a marine fish. Proc Biol Sci 274: 1693-1699.
- Conover DO, Baumann H (2009) The role of experiments in understanding fishery-induced evolution. Evol Appl 2: 276–290.
- Shaw ES, Aronson LR (1954) Oral incubation in Tilapia macrocephala. 1, Embryological studies. Bulletin of the AMNH 103: 5.
- R Development Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
- Adépo-Gourène B, Pouyaud L, Teugels GG, Hansen MM, Agnèse JF (1998) Morphological and genetic differentiation of West African populations of Sarofherodon melanotheron Ruppel, 1852 (Teleostei, Cichlidae. Paris : ORSTOM 189-198.
- Ndiaye A (2012) Réponses du tilapia Sarotherodon melanotheron aux stress multiples (salinité et contaminants chimiques): approche multi-paramétrique. Thèse de doctorat, Université Montleiier II: 230.
- Yoboué AN, Adepo-Gourène BA, Séka D, Durand JD, Panfili J, et al. (2012) Genetic diversity and adaptability of Sarotherodon melanotheron (Cichlidae) in coastal ecosystem. Ethology Ecology & Evolution iFirst 24: 230-243.
- Watanabe WO, Kuo CM (1985) Observations on the reproductive performance of Nile tilapia Oreochromis niloticus in laboratory aquaria at various salinities. Aquaculture 49: 315-323.
- Gilles S, Zamora L, Amiel C, Rodriguez JL (2004) Comparative study of reproductive characteristics of the euryhaline tilapia, Sarotherodon melanotheron heudelotii in fresh and sea waters. Journal of Aqua Trop 19: 277-284.
- Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford, UK.
- Duponchelle F, Legendre M (2001) Rapid phenotypic changes of reproductive traits in response to experimental modifications of spatial structure in Nile tilapia, Oreochromis niloticus. Aquatic Living Resources 14: 145-152.
- Legendre M, Trebaol L (1996) Efficacité de l’incubation buccale et fréquence de ponte de Sarotherodon melanotheron en milieu d’élevage (lagune Ébrié, Côte d’Ivoire). In: 161 Pullin RSV, Lazard J, Legendre M, Amon Kothias JB, Pauly D (Eds.). Proceedings of the 3 rd international symposium on tilapia in aquaculture. ICLARM, Manila 375-386.
-
-
-
-









Table 1:
Daily variations in physicochemical parameters during the experimentation (Test-U: ns = no significant at probability level 0.05).