Authors:
Martin H Teicher1,2*, Danielle M Webster3 and Steven B Lowen3
1Department of Psychiatry, Harvard Medical School, USA
2Department of Psychiatry, Harvard Medical School, USA
3Department of Psychiatry, Harvard Medical School, USA
Received: 17 May, 2017; Accepted: 25 May, 2017; Published: 26 May, 2017
Martin H Teicher, Mclean Hospital, Developmental Biopsychiatry Research Program, 115 Mill Street, Belmont, MA 02478-9106, USA, Tel: (617) 855-2970; Fax: (617) 855-3712; E-mail:
Teicher MH, Webster DM, Lowen SB (2017) Bupropion Sustained released versus Placebo for seasonal affective Disorder. Arch Depress Anxiety 3(1): 009-013. DOI: 10.17352/2455-5460.000016
© 2017 Teicher MH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
SAD; Winter depression; Bupropion; Gender differences
Background: The majority of seasonal affective disorder (SAD) studies have evaluated the use of light or selective serotonin reuptake inhibitors (SSRI). The purpose of the present study was to evaluate bupropion sustained-released (SR), a non-SSRI antidepressant, for the treatment of SAD.
Method: Forty-one adults meeting DSM IV criteria for SAD were recruited into a six-week, randomized, double-blind, placebo-controlled trial. Participants started on bupropion SR 150 mg QD (or equivalent placebo pills) and titrated up to 200 mg BID if tolerated by week 4. Participants were evaluated weekly with SIGH-SAD and self-reported Kellner Symptom Questionnaire (SQ). Mixed effects growth models and receiver operating characteristic (ROC) analyses were used to compare treatments.
Results: Analysis was done on the 36 participants who completed at least 2 weeks of treatment, as per protocol. Thirty-four participants completed the entire protocol; two participants receiving placebo dropped out during weeks 3 and 5. Sixteen participants (7 male, 9 female, 46.5 + 9.6 years, mean+SD) received bupropion SR and 20 participants (8 males, 12 females, 48.2+8.8 years) received placebo. Participants receiving bupropion SR had a more rapid reduction in atypical SIGH-SAD depressive symptoms and lower depression scores across time on the SQ. ROC analyses revealed that positive effects of bupropion SR on total SIGH-SAD scores were more evident in males than females. Bupropion SR was well tolerated.
Conclusion: Bupropion SR may be beneficial for the treatment of SAD, but larger randomized placebo-controlled studies are warranted.
Introduction
Recurrent major depression with seasonal pattern (SAD) may affect between 1% and 3% of adults living far from the equator [1]. Treatment studies have focused on the use of light. However, many patients find light therapy inconvenient, or require medications as sole or adjunctive treatment. Relatively few controlled medication trials have been reported. Briefly, several agents have been found to be ineffective. These include atenolol [2], as a suppressor of melatonin production, melatonin itself [3], levodopa plus carbidopa [4] and cyanocobalamin [5], as an agent that facilitates entrainment of circadian rhythms. Preliminary positive results have been obtained with d-fenfluramine [6], low-dose morning propranolol [7] and tryptophan [8,9]. Randomized double-blind placebo controlled trials of conventional antidepressants have demonstrated modest effects. Moclobemide (a reversible MAO-A inhibitor) decreased atypical depression scores compared with placebo but had no significant effect on the Montgomery and Asberg Depression Rating Scale or Clinical Global Improvement scores [10]. Using the 29-item modified Structured Hamilton depression Rating Scale (SIGH-SAD) [11], Lam et al. [12], found that fluoxetine was significantly more effective than placebo based on the percent of participants with a 50% or greater reduction in depression scores (59% vs. 34%), but not in terms of average depression scores. A large-scale Canadian study found that fluoxetine (20 mg/d) was as effective as 10,000 lux light therapy, but interpretation is clouded by lack of a placebo arm [13]. Moscovitch et al. [14], reported superiority of sertraline over placebo producing a 52% vs. 43% reduction in Hamilton Depression Ratings.
An open trial of bupropion for SAD reported marked efficacy [15]. Since many patients with SAD have symptoms associated with atypical depressions including psychomotor retardation, carbohydrate craving, and hypersomnia, and may have a mild form of bipolar disorder [16], bupropion appears to be a good theoretical choice [17,18]. Bupropion was evaluated in a large multicenter international study for its potential to prevent recurrence of SAD when initiated prior to the fall-winter season. Bupropion produced a 44% relative risk reduction versus placebo [19] and the FDA subsequently approved bupropion for this purpose. Part of the scientific rationale for conducting a trial of bupropion for prophylaxis of SAD was an unpublished randomized double-blind placebo controlled treatment trial. This is a report of the results of that study conducted during the fall/winter 1997/98 and 1998/99.
Methods
All participants gave written informed consent for this IRB-approved study after procedures and possible side effects were fully explained. Participants were recruited from the community by newspaper advertisements.
The study was designed as a double-blind, randomized, parallel group placebo control trial. Entry requirements included a diagnosis of Major Depression, Recurrent with Seasonal Pattern based on DSM-IV and Rosenthal–NIMH criteria [16]. Briefly, these criteria require that individuals have a history of recurrent depressive episodes that regularly recur and remit during specific times of the year, this pattern must have lasted at least two years with no nonseasonal episodes and that the number of seasonal episodes substantially outnumber any non-seasonal episodes. Participants also had to have a baseline 29-item Structured Interview Guide for the Hamilton Depression Scale with Atypical Depression Supplement (SIGH SAD) score of at least 20, to be medically healthy and on no medications except hormone replacement therapy (HRT) or oral contraceptives. Participants also had to indicate that they had no plans to travel to tropical locations during the time that there were enrolled in the study. Individuals with anorexia, bulimia or history of seizures were excluded. Potential participants who met these criteria then received a blood test to assess thyroid status and returned 1-2 weeks later for reassessment. On the second baseline assessment they also needed to have a SIGH-SAD score of at least 20 and normal thyroid profile. Those who did were randomized to drug or placebo.
Efficacy measurements included interviewer-based SIGH SAD scores [11] and self-reported symptoms of depression on Kellner’s Symptom Questionnaire (SQ) [20]. Potential adverse events were assessed each week by inquiring about specific side effects including dry mouth, nausea, weight loss, insomnia, anxiety, agitation, seizures, dizziness and constipation.
Following the second baseline measure participants received 6 weeks of treatment on drug or placebo. Bupropion SR was introduced at 150 mg once daily, and titrated to 200 mg twice daily by week 4, if tolerated. Participants receiving placebo followed the same titration schedule.
Placebo-controlled trials of light therapy indicate that conventional analysis of mean improvement on SIGH-SAD scores often fails to detect a significant light-placebo difference [21,22]. Light therapy emerges as superior to placebo, based on percentage of participants showing full clinical remission, and by Receiver-Operating Characteristic (ROC) analysis of the data [22]. Unlike conventional statistical tests, ROC analyses make no assumptions about the distribution of the data or the scaling properties of the measure. It also does not force the investigator to make an arbitrary decision that a specific score or percent change criteria is required for improvement or remission. Rather, it portrays the differential pattern of response of patients on drug versus placebo across the entire range of scores. Assuming that there was no difference between drug and placebo then the area under the ROC curve (AUC ROC) would be 0.5, and the ROC curve would follow a straight line from the origin (0,0) to the joint maximum (1,1). Comparing the actual pattern of drug-placebo response to the theoretical null effect line provides a powerful means of detecting a differential pattern of response to drug, and also provides novel insight into the type of therapeutic response obtained. ROC response was analyzed in two ways. First we assessed the significance of the area under the curve, which is equivalent to the Mann–Whitney U test or Wilcoxon rank sum test. Second, we used the Kolmogorov-Smirnov test [23], to examine maximal degree of departure from the null effect chance line to determine if there was a differential pattern of response to drug versus placebo.
The Fisher Exact Test was used to compare percentage of participants meeting remission criteria at endpoint on drug versus placebo. Finally, hierarchical growth models [24], were used to compare drug versus placebo differences in time course of response based on ratings at baseline and during each of the 6 treatment weeks.
Because the sample size was modest and this was a preliminary study we used a directional one-tailed criteria P1T < 0.05 testing whether participants on bupropion showed a superior response than participants on placebo, or two-tailed directionless criteria of P < 0.1.
Results
Forty-six participants were recruited and came in for their first baseline assessment. Five were excluded from the study (2 due to elevated TSH levels, 3 for work schedule or vacation conflicts) prior to medication assignment. Of the 41 patients to begin the protocol, 5 dropped out (3 on medication, 2 on placebo) before completing two weeks of treatment (2 sought other treatment, 3 experienced side effects). Thirty-six participants who completed at least two weeks of treatment were included in the analyses, as per protocol. Thirty-four of the 36 participants completed all six weeks of treatment. Two participants on placebo withdrew from the study. One participant with minimal placebo response withdrew at week three because of side effects. The other with a moderate placebo response was withdrawn at week five due to an unrelated medical problem. The last observations from these participants were carried forward to endpoint. Sixteen participants (7 males, 9 females, 46.5 ± 9.6 years, mean ± SD) received bupropion SR for 6 weeks and 20 participants (8 males, 12 females, 48.2 ± 8.8 years) received placebo for an average of 5.8 weeks. Initial group description is shown in table 1.
At endpoint, full clinical remission (50% or greater decrease in SIGH-SAD scores and final score of < 8) occurred in n=4 (20%) participants on placebo and in n=7 (44%) participants on bupropion SR. Hence, the Odds Ratio for remission on drug versus placebo was 3.00 {95% CI 0.6 – 18.2}, but was not significant given the limited sample size (Fisher exact P1T = 0.12). Hierarchical growth curve analysis indicated that the SIGH-SAD total scores were better fit by a 2-component piecewise growth model with first-order autoregressive (AR1) correlation than by a 1 component AR1 model (LR = 49.3, P < 0.0001), linear model (LR = 64.92, P <0.0001), or quadratic model (LR = 25.7, P < 0.0001). As seen in figure 1 there was a marked drop in SIGH-SAD scores from baseline to first post-treatment visit (T1: F1,212 = 48.50, P < 0.0001) and a gradual reduction from first to sixth post-treatment visit (T2: F1,212 = 30.33, P < 0.0001). However, there was no significant overall difference between participants receiving bupropion versus placebo (F1,33 = 0.29, P > 0.5) nor any significant treatment by T1 (FF1,212 = 1.85, P = 0.17) or treatment x T2 interactions (F1,212 = 1.72 P = 0.19).
-
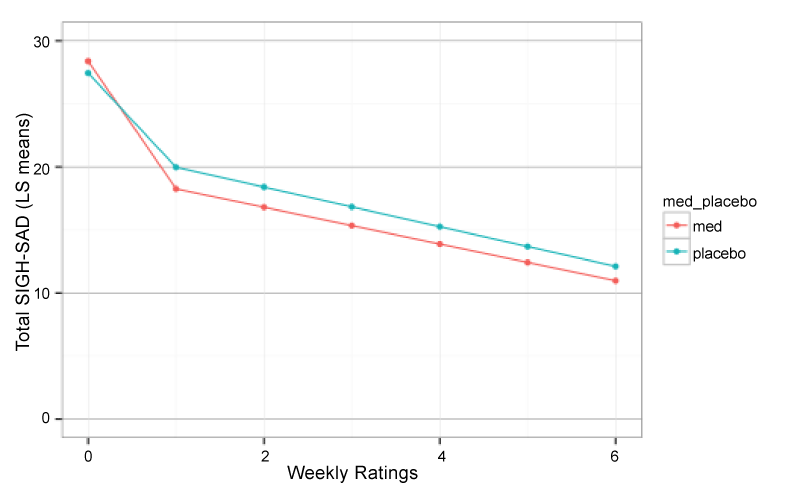
Figure 1:
Piecewise hierarchical growth model showing least square mean total depression scores (SIGH-SAD) across time in participants receiving bupropion-SR versus placebo. Week 0 is their unmedicated baseline score followed by six successive weeks on bupropion or placebo.
Total SIGH-SAD score represent the sum of the standard 21-item Hamilton Depression Rating Scale (HDRS-21) plus an 8-item atypical addendum that includes features such as hypersomnia, carbohydrate craving and reverse diurnal variation. These “atypical” features are included in the SIGH-SAD as they tend to better characterize individuals with seasonal depression than more typical depressive symptoms. There was no evidence for medication versus placebo differences on HDRS-21 scores; however there were significant T1 x treatment (F1,212 = 3.75, P = 0.05) and T2 x treatment (FF1,212 = 3.19, P < 0.08) interactions on atypical depression scores (Figure 2).
-
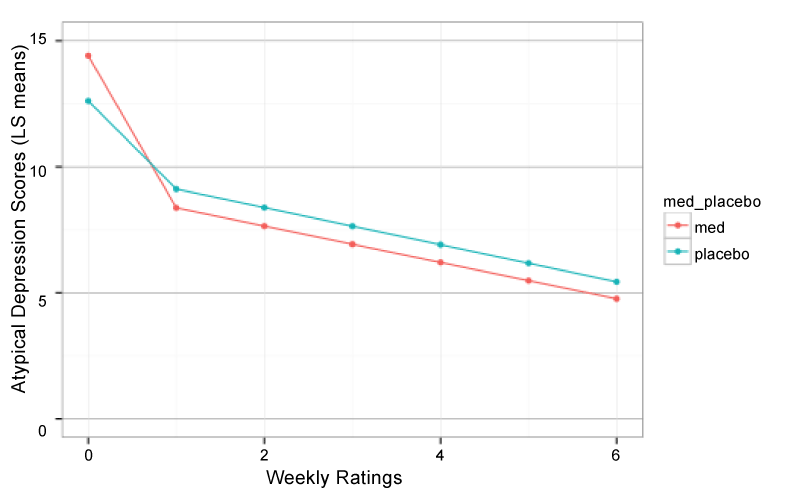
Figure 2:
Piecewise hierarchical growth model showing least square mean atypical depression scores (8 items) across time in participants receiving bupropion-SR versus placebo.
As seen in figure 3, AUC ROC for final SIGH-SAD scores (AUC = 0.548, p = 0.32) was not significant, but there was evidence for a differential pattern of response. The ROC curve deviated significantly from the null effect line with the greatest deviation occurring around final SIGH-SAD scores of 7 (P < 0.05). ROC analysis demonstrated distinctly different drug response patterns in males vs. females. As illustrated in figure 3, the ROC curve for males departed significantly from the null effect line, particularly at scores of 7-9. All male participant scores between 4-7 (remission) occurred on drug while all scores between 18-28 (no benefit) occurred on placebo. In contrast, females showed a divergent response pattern. Virtually all scores between 10-16 (partial response) occurred in women on placebo. Nearly all scores between 3 - 7 (remission) and nearly all scores between 17 - 26 (no benefit) occurred on bupropion SR. Hence, women receiving bupropion SR either did quite well or reported more symptoms than women receiving placebo.
-
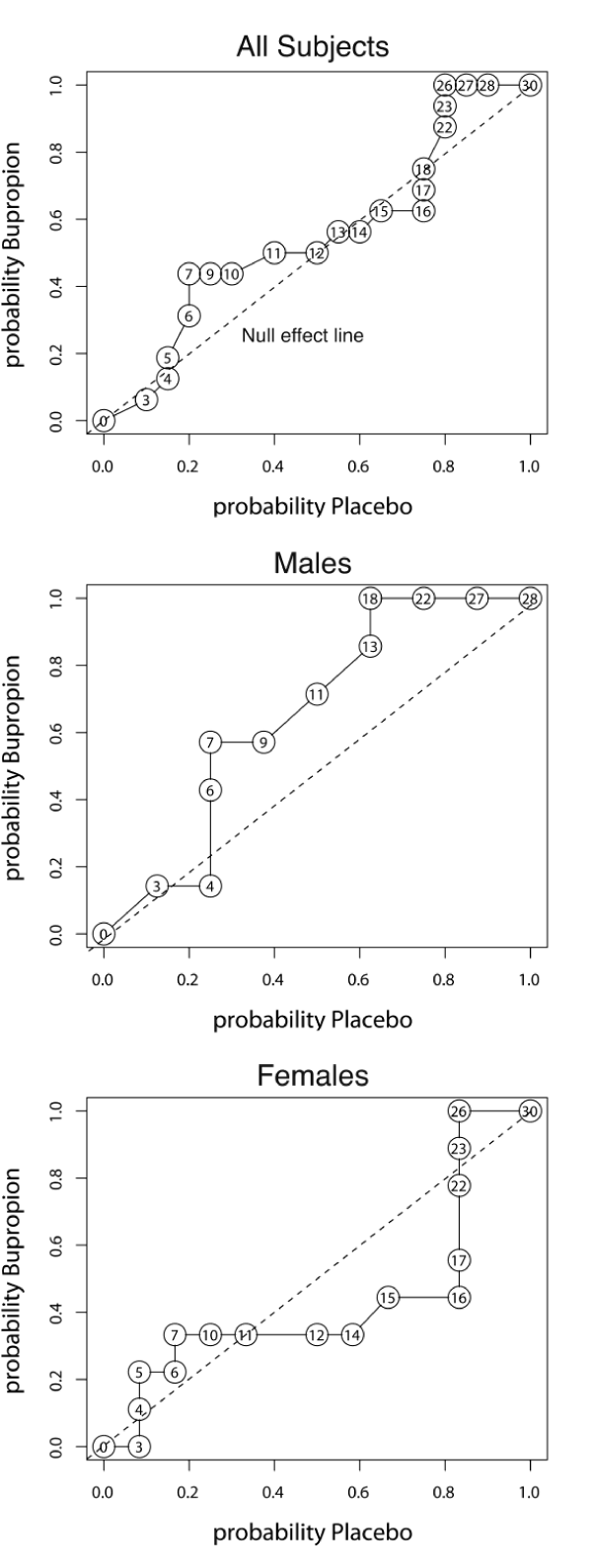
Figure 3:
Receiver operating characteristic (ROC) analysis of total SIGH-SAD scores at endpoint in entire sample and in male and female subsamples. The dotted line in each graph is the null effect line. There was no significant overall benefit of bupropion on SIGH-SAD scores for the entire sample based on area under the ROC curve. However, there was a differential pattern of response to bupropion versus placebo based on Kolmogov-Smirnof analyses, as the distribution of probability scores deviated significantly from the chance -null effect line around SIGH-SAD scores of 7. Males and females showed different patterns of responses. Men showed a potential drug advantage, with a ROC area of 0.652. Men deviated significantly from the null effect line, particularly at scores of 7-9 and 18-22. In contrast, women showed no overall benefit on bupropion, as some did quite well but others had scores higher than women taking placebo. Number in circles are total SIGH-SAD depression scores at each point.
There was evidence for a therapeutic effect on the hierarchical piecewise growth curve analysis of self-reported SQ depression scores (F1,34 = 2.95, P = 0.095) indicating that participants receiving bupropion had lower mean scores across time, but there were no significant time x treatment interactions. ROC analysis of SQ depression scores showed trend-level drug placebo difference in final scores (AUC ROC = 0.639, P1T < 0.08). The Kolmogorov-Smirnov test showed clear evidence for a significant departure from the null effect line at final SQ scores of 5 (P < 0.02), indicating a differential pattern of response with more participants on bupropion showing very low depression scores than on placebo.
In comparison with other studies that asked “Have you felt different in any way since your last visit?,” we specifically inquired about common side effects each week. Overall bupropion SR was well tolerated. The only significant side effect was constipation reported by 6 (37%) participants on bupropion SR and 2 (10%) on placebo (Fisher Exact, P1T = 0.058). Interestingly 5 out of 9 females on bupropion SR reported this as a side effect, but constipation was not related to degree of antidepressant response.
Discussion
The results of this small randomized, double-blind, placebo-controlled study were consistent with a previous open trial study of bupropion in SAD [15]. Participants receiving bupropion SR in the current study showed more rapid reduction in atypical depression scores and lower mean levels of self-reported SQ depression across ratings. All of the other findings, such as the 3-fold greater odds on bupropion versus placebo of showing a complete recovery, were in the right direction, but non-significant given the limited sample size. The finding that there were significant time by treatment interactive effects on atypical but not typical depression scores was consistent with result reported by Lingjaerde et al. [10], using moclobemide.
It was also interesting that males and females displayed different response patterns on ROC analysis of final SIGH-SAD depression ratings. Bupropion SR was associated with low scores and placebo with high scores in males. Women receiving bupropion SR clustered into two groups. Some had a good therapeutic response while others were more symptomatic than women receiving placebo. Conventional analyses comparing average group scores or percent remission rates would fail to delineate these differential response patterns. It should be noted that the ROC curve for female SIGH-SAD scores was unusual, and proper ROC curves for signal detection never have a concave upward appearance. However, this is not necessarily true using ROC to analyze drug effects, as medications can produce both improvement and worsening in a group of participants. A Monte-Carlo simulation of mixed response to drug confirmed that ROC curves of this shape arise, as predicted. Hence, these unusual ROC curves are consistent with mixed response to medication.
One possibility is that bupropion SR may exert a more robust catecholaminergic response in males than females due to the complex effects of estrogen on catecholamine neurotransmission [25]. Although there were 5 women on bupropion SR who had reached menopause, all but one received hormone replacement therapy. Three women age 50 and over had an excellent response to bupropion while four had a minimal response. Younger women had an intermediate response. These results are compatible with a re-analysis of antidepressant studies that found that depressed men have a better therapeutic response to imipramine than depressed premenopausal women [26].
Study limitations include a 6-week treatment duration with only 3 weeks on bupropion SR at 200 mg BID. The milder winter seasons (fall/winter 97/98 and 98/99) may have affected our participant population and severity of reported symptoms. Most important, the sample size was small, especially for male and female subgroup analysis. Nevertheless, this pilot study warrants further evaluation in larger controlled trials.
Acknowledgments
Supported, in part, by Glaxo Wellcome, Inc, now GlaxoSmithKline, as an investigator initiated study. The company played no role in the design, analysis or reporting of results. We thank Carol A. Glod PhD for assistance in subject recruitment and assessment.
-
-
- Levitt AJ, Boyle MH, Joffe RT, Baumal Z (2000) Estimated prevalence of the seasonal subtype of major depression in a Canadian community sample. Can J Psychiatry 45: 650-654. Link: https://goo.gl/PHh9Jy
- Rosenthal NE, Jacobsen FM, Sack DA, Arendt J, James SP, et al.,. (1988) Atenolol in seasonal affective disorder: a test of the melatonin hypothesis. Am J Psychiatry 145: 52-56. Link: https://goo.gl/M0aL2E
- Wirz JA, Graw P, Krauchi K, Gisin B, Arendt J, et al.,. (1990) Morning or night-time melatonin is ineffective in seasonal affective disorder. J Psychiatr Res 24: 129-137. Link: https://goo.gl/ln9EW0
- Oren DA, Moul DE, Schwartz PJ, Wehr TA, Rosenthal NE (1994) A controlled trial of levodopa plus carbidopa in the treatment of winter seasonal affective disorder: a test of the dopamine hypothesis. J Clin Psychopharmacol 14: 196-200. Link: https://goo.gl/e6rwVW
- Oren DA, Teicher MH, Schwartz PJ, Glod C, Turner EH, et al.,. (1994) A controlled trial of cyanocobalamin (vitamin B12) in the treatment of winter seasonal affective disorder. J Affect Disord 32: 197-200. Link: https://goo.gl/KNbj9y
- O'Rourke D, Wurtman JJ, Wurtman RJ, Chebli R, Gleason R (1989) Treatment of seasonal depression with d-fenfluramine. J Clin Psychiatry 50: 343-347. Link: https://goo.gl/Z8Wt8v
- Schlager DS (1994) Early-morning administration of short-acting beta blockers for treatment of winter depression. Am J Psychiatry 151: 1383-1385. Link: https://goo.gl/7pxCBQ
- Ghadirian AM, Murphy BE, Gendron MJ (1998) Efficacy of light versus tryptophan therapy in seasonal affective disorder. J Affect Disord 50: 23-27. Link: https://goo.gl/aqk58g
- McGrath RE, Buckwald B, Resnick EV (1990) The effect of L-tryptophan on seasonal affective disorder. J Clin Psychiatry 51: 162-163. Link: https://goo.gl/MdXpt2
- Lingjaerde O, Reichborn-Kjennerud T, Haggag A, Gartner I, Narud K, et al.,. (1993) Treatment of winter depression in Norway. II. A comparison of the selective monoamine oxidase A inhibitor moclobemide and placebo. Acta Psychiatr Scand 88: 372-380. Link: https://goo.gl/YlUmS5
- Williams JBW, Link MJ, Rosenthal NE, Terman M (1988) Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGHSAD). New York: New York Psychiatric Institute. Link: https://goo.gl/TBt27i
- Lam RW, Gorman CP, Michalon M, Steiner M, Levitt AJ, et al.,. (1995) Multicenter, placebo-controlled study of fluoxetine in seasonal affective disorder. Am J Psychiatry 152: 1765-1770. Link: https://goo.gl/pqSH3J
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, et al.,. (2006) The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry 163: 805-812. Link: https://goo.gl/am1TFa
- Moscovitch A, Blashko CA, Eagles JM, Darcourt G, Thompson C, et al.,. (2004) A placebo-controlled study of sertraline in the treatment of outpatients with seasonal affective disorder. Psychopharmacology 171: 390-397. Link: https://goo.gl/JUR0lB
- Dilsaver SC, Qamar AB, Del Medico VJ (1992) The efficacy of bupropion in winter depression: results of an open trial. J Clin Psychiatry 53: 252-255. Link: https://goo.gl/Q7rPdz
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, et al.,. (1984) Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41: 72-80. Link: https://goo.gl/nEMqms
- Goodnick PJ, Dominguez RA, DeVane CL, Bowden CL (1998) Bupropion slow-release response in depression: diagnosis and biochemistry. Biol Psychiatry 44: 629-632. Link: https://goo.gl/0DZGjM
- Frances AJ, Kahn DA, Carpenter D, Docherty JP, Donovan SL (1998) The Expert Consensus Guidelines for treating depression in bipolar disorder. J Clin Psychiatry 59: 73-79. Link: https://goo.gl/pd2I16
- Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, et al.,. (2005) Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry 58: 658-667. Link: https://goo.gl/dFtSXS
- Kellner R (1987) A symptom questionnaire. Journal of Clinical Psychiatry 48: 268-273. Link: https://goo.gl/RGt0iF
- Terman M, Terman JS, Ross DC (1998) A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry 55: 875-882. Link: https://goo.gl/bj4rU6
- Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM (1998) Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry 55: 883-889. Link: https://goo.gl/sgWNFs
- Siegel S (1956) Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill. Link: https://goo.gl/JMBTJu
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2015) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3: 1-120.
- Gordon J, Perry K (1983) Pre- and postsynaptic neurochemical alterations following estrogen-induced striatal dopamine hypo- and hypersensitivity. Brain Research Bulletin 10: 425-428. Link: https://goo.gl/JIawt7
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB (2000) Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 157: 1445-1452. Link: https://goo.gl/FHSbhM









Table 1:
Participant characteristics.
Measures
Bupropion
Placebo
t-test
df
p
AGE
46.50 ± 9.65
47.95 ± 8.77
-0.47
30.77
p>0.6
SQ Anxiety
8.38 ± 5.24
11.10 ± 5.75
-1.48
33.37
p>0.1
SQ Depression
9.94 ± 5.32
12.90 ± 5.49
-1.64
32.69
p>0.1
SQ Somatization
8.81 ± 4.90
8.40 ± 3.76
0.28
27.62
p>0.7
SQ Anger-Hostiity
9.38 ± 5.32
9.35 ± 5.44
0.01
32.60
p>0.9
SIGH-SAD Typical
13.34 ± 4.07
14.13 ± 3.44
-0.61
29.44
p>0.5
SIGH-SAD Atypical
15.59 ± 4.06
13.98 ± 3.79
1.22
31.19
p>0.2
SIGH-SAD Total
28.94 ± 4.85
28.10 ± 5.33
0.49
33.39
p>0.6
SQ – Kellner Symptom Questionnaire.