Author(s):
Sumedha Sirohi1, Sumit Bhutani1, Cherry Cheema1, Gurleen Kaur2, S P Singh3, Prithpal S Matreja4*
1MBBS Student, Fourth Year, Gian Sagar Medical College and Hospital, Village Ram Nagar, District Patiala, Punjab, India-140601
2Assistant Professor, Department of Pharmacology, Gian Sagar Medical College and Hospital, Village Ram Nagar, District Patiala, Punjab, India-140601
3Professor & HOD, Department of Dermatology, Gian Sagar Medical College and Hospital, Village Ram Nagar, District Patiala, Punjab, India-140601
4Professor and Head, Department of Pharmacology, Teerthankar Mahaveer Medical College & Research Center, TMU, Moradabad, 244001, Uttar Pradesh, India
Received: 07 July, 2017; Accepted: 07 September, 2017; Published: 09 September, 2017
Prithpal Singh Matreja, Department of Pharmacology, Professor and Head, TeerthankarMahavir Medical College Research Center, TMU Moradabad, UP, India, Tel: +91-9855001847; E-Mail:
Sirohi S, Bhutani S, Cheema C, Kaur G, Singh SP, et al. (2017) Comparison of safety and efficacy of Oral Terbinafine with Amphotericin B gel and Sertaconazole cream for the treatment of Tinea Corporis and its effect on quality of life of patients. Int J Dermatol Clin Res 3(1):018-021. DOI: 10.17352/2455-8605.000021
© 2017 Sirohi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Dermatophystosis; Cutaneous; Erythema; Itching; Scaling
Background: Tinea Corporis is a superficial fungal infection affecting 20-25% of the world’s population. Management involves using topical and oral antifungal drugs. Terbinafine has been used commonly for the treatment of tinea corporis to prevent the emergence of resistance combination of drugs is used. Hence, this study was designed to compare the safety and efficacy of Sertaconazole and Amphotericin B when either is used with oral Terbinafine.
Methodology: This open-label, randomized, prospective and parallel group study was conducted on patients suffering from tinea corporis visiting the Department of Dermatology. All patients underwent a thorough clinical examination to establish the diagnosis of tinea corporis and were divided into two groups. Patients in Group A received treatment with Sertaconazole and Terbinafine. Group B received treatment with Amphotericin B and Terbinafine. At the end of treatment phase patients were assessed clinically for the disease symptoms - erythema, pruritus, and scaling; adverse effects experienced and Dermatological life quality index (DLQI).
Results: Fifty two (52) patients were enrolled in the study, both groups were comparable at baseline. After 2 weeks of treatment, complete clinical cure was found in 6 patients in Group A and 5 patients in Group B. The treatment also showed improvement in clinical disease activity as there was clinical improvement seen in 22 patients in group A as compared to 25 patients in Group B. Greater number of patients in Group B reported with symptomatic improvement. There was no serious adverse event reported in both groups and DLQI score was comparable.
Conclusion: The use of topical antifungals along with systemic antifungals has been found to be useful in early achievement of clinical cure and improvement in clinical symptoms of tinea corporis.
Introduction
Tinea Corporis is a superficial fungal infection (dermatophytosis) anywhere on the body except the scalp, beard, feet, or hands. Fungal infections of the skin and nails have been found to affect 20-25% of the world’s population, making them one of the most frequent forms of infection [1]. It may occur on any part of the body, and may have a variety of appearances, most easily identifiable by enlarging raised red rings with a central area of clearing [2]. The disease can be acquired by person to person transfer usually via direct skin contact with affected individual. The fungus also spreads by touching inanimate objects like personal care products, bed linen, combs, athletic gear or hair brushes contaminated by affected person [2]. Generally a mild and non- threatening condition, tinea can affect people at any age. Individuals at high risk include those living in crowded or humid conditions, sweat excessively, wear tight constrictive clothing with poor circulation, and have weakened immune system. The quality of life has been shown to be impaired with tinea corporis [3].
The pharmacological management of tinea corporis involves using topical and oral antifungal drugs like allylamines, azoles, polyene antibiotics. The topical agents are more commonly used; the oral drugs being used for extensive lesions [4,5].
Out of the various antifungals, terbinafine and itraconazole are commonly prescribed drugs. A meta-analysis by Rotta et al, found terbinafine (allylamine) to be superior to azoles group of antifungal drugs [6]. Sertaconazole, an imidazole antifungal drug, inhibits the synthesis of ergosterol and posses potent anti-inflammatory action also [4, 7]. A prospective, single blind, randomized control trial done to compare topical sertaconazole with topical terbinafine showed that at the end of 3 weeks both terbinafine and sertaconazole groups had complete cure. Sertaconazole was as effective as terbinafine and both drugs showed good tolerability with no adverse effects [7]. Another study done to compare efficacy of Sertaconazole and Clotrimazole in Tinea corporis patients showed significant reduction in erythema, itching, and margins of lesion (p<0.001) among Sertaconazole group within a span of three weeks in the treatment [8].
A study done to assess the safety, tolerability and efficacy in mucocutaneous fungal infection of amphotericin B gel showed that appreciable number of patients was cured at the end of treatment with no serious adverse effect reported [9]. Lipid based Amphotericin B gel formulation is more compatible with skin, showed encouraging clinical results and has lesser adverse drugs reactions like; blistering, itching, redness, peeling, dryness or irritation of the skin [4,9].
Terbinafine has been used commonly for the treatment of tinea corporis but in recent years, the resistance has been increasing. To prevent the emergence of resistance and wide coverage, combination of drugs from different groups of antifungals should be used [4,5,10].
Not many studies are there to compare the combination therapy of oral with topical agents in the treatment of tinea corporis. Most of the data till date available about the treatment of the disease is mainly from studies done in other parts of world. Hence, this study was designed to compare the safety and efficacy of Sertaconazole cream and Amphotericin B gel when either is used along with oral Terbinafine. Also, this study assessed the improvement in quality of life of patients of tinea corporis after treatment with these drugs.
Material and Methods
This open-label, randomized, prospective and parallel group study was conducted in the outpatient department of dermatology in a tertiary care hospital after approval from Institutional Ethics Committee (IEC) for duration of 2 months from April to May 2016. A total of 52 patients suffering from tinea corporis visiting the Department of dermatology were enrolled in the study after they gave a written informed consent. The clinical diagnosis of tinea corporis was made based upon the presenting signs and symptoms of the patient.
Patients clinically diagnosed with tinea corporis infection, of either sexes and less than 60 years of age were included in the study. All patients enrolled in the study had positive skin scraping under direct microscopy.
Patients suffering from diabetes mellitus, sub acute cutaneous lupus erthematosus [11], clinical cases of eczema, lichen planus, drug induced eruptions, urticaria, tinea ungium and any chronic medical illness were excluded from the study. Patients with known history of hypersensitivity to any component of drug, substance abuse, pregnant and lactating mothers were also excluded from the study.
Procedure
All patients underwent a thorough clinical examination to establish the diagnosis of tinea corporis and were divided into two groups. Patients in Group A received treatment with Sertaconazole (2%) cream applied topically on the affected area twice daily and Terbinafine (250 mg oral) once daily for two weeks. Group B received treatment with Amphotericin B (1%) gel applied twice daily on the affected area and Terbinafine (250 mg oral) once daily for two weeks. At the end of treatment phase, there was a follow-up phase at the end of two weeks where the patients were assessed clinically for the disease symptoms. The parameters of clinical disease activity (erythema, pruritus, scaling) were assessed and scored as: 0 = absent; 1 = mild; 2 =moderate; 3 = severe.
Complete cure of the disease was judged when no residual clinical signs and symptoms were there and effective therapy was adjudged when mild residual clinical signs and symptoms were present.
The safety of the combination drugs was assessed by noting adverse effects, which occurred during and at the end of treatment. If any, their severity was graded as mild, moderate or severe. Then, they were asked to fill a DLQI form to assess the effect of the diseaseon quality of life of these patients.
Primary outcome measures
➢ Clinically by assessing symptomatic relief in pruritus, erythema and scaling
➢ Adverse effects experienced by the patients
➢ Dermatological life quality index (DLQI)
Dermatology Life Quality Index (DLQI)
It is a questionnaire to measure how much skin problem has affected the life of the patients. The DLQI questionnaire is designed for use in adults, i.e. patients over the age of 16. It is self-explanatory and can be simply handed to the patient who can fill it without the need for detailed explanation. It is usually completed in 1-2 minutes. Each question has a score range from 0-3.The DLQI is calculated by summing the score of each of the 10 questions resulting in a maximum of 30 and a minimum of 0. The higher the score, the more the quality of life is impaired. The DLQI can also be expressed as a percentage of the maximum possible score of 30. The scoring pattern is:
1. 0-1: no effect at all on patient’s life
2. 2-5: small effect on patient’s life
3. 6-10: moderate effect on patient’s life
4. 11-20: very large effect on patient’s life
5. 21-30: extremely large effect on patient’s life [12-14].
Statistical analysis
The data was tabulated as mean ± standard deviation (SD). Results were analyzed using various non-parametric tests (ChiSquare Test) and parametric tests (two-tailed student t-test). A p<0.05 was considered statistically significant.
Results
Fifty two (52) patients were enrolled after they gave their written informed consent. The baseline parameter were comparable in both the groups at baseline, no significant difference was found in gender, age, and duration of disease and family history of the disease between the two groups, indicating similar demographic profile of the patients in the two groups (Table 1).
Treatment effectiveness
After 2 weeks of treatment, complete clinical cure was found in 6 patients in Group A and 5 patients in Group B. The treatment also showed improvement in clinical disease activity as there was clinical improvement seen in 22 patients in group A as compared to 25 patients in Group B (Figure 1). The difference between the efficacy in the two groups was statistically insignificant with p-value of 0.16.
-
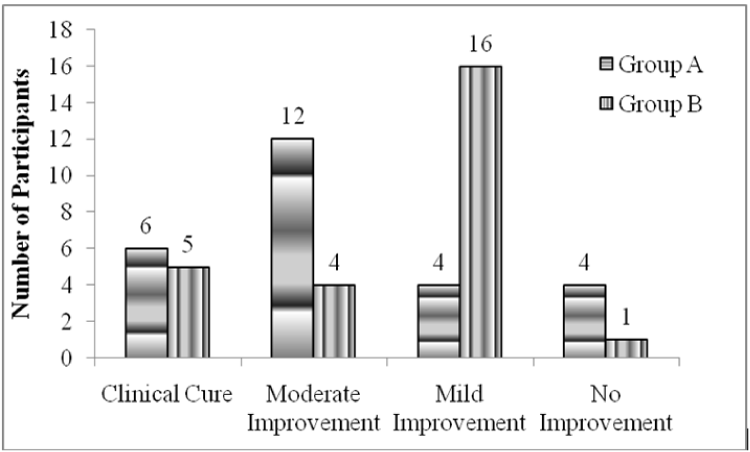
Figure 1:
Clinical Improvement in both groups.
Greater number of patients in Group B reported with symptomatic improvement though it was not statistically significant. Erythema in two groups, it was seen that redness was resolved in 7 and 11 patients of Group A and Group B respectively. Similarly, improvement in itching showed 10 and 12 patients with 100% resolved symptom in the group A and Group B respectively (Figure 2). Similarly more number of patients had improvement in scaling in Group B (Figure 2).
-
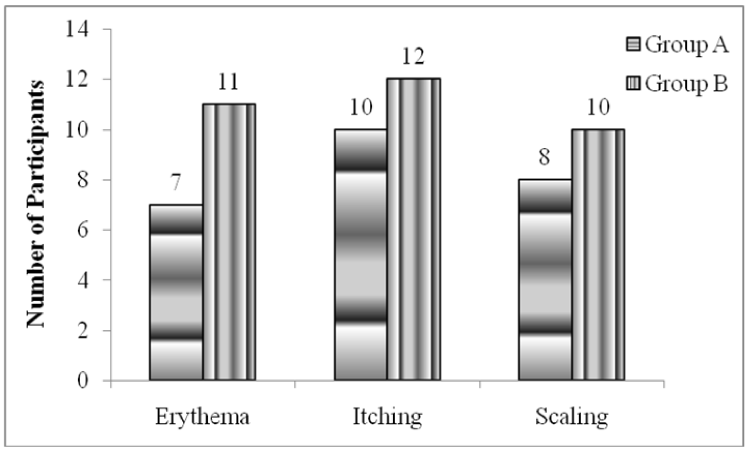
Figure 2:
Symptomatic Improvement in both groups.
Safety profile
There was no serious adverse event reported in both groups. None of the patients required reduction in dose or any therapy for treatment of adverse events. No patient was lost to the follow up.
DLQI score
The mean ± SD DLQI score in Group A and Group B was 7.38 ± 2.91 and 7 ± 2.48 respectively at the end of two weeks of treatment. There was statistically insignificant difference between the DLQI scores in both groups.
Discussion
Despite the increasing prevalence of cutaneous dermatophytosis across the world, and especially in tropical countries like India, the data on guidelines on the management of tinea corporis are not adequate [4]. Few Cochrane reviews on topical therapy in tinea corporis have helped to bridge this knowledge gap but still some guidelines/ trials on the dose and duration of the combination of systemic and oral antifungal in tinea corporis are conspicuous by their absence [4,10].
Our study did not find any significant difference in the age, gender, age, and duration of disease and family history of the disease. A similar study with no significant differences in the age, sex, duration of disease, family members with fungal infections found 34 years as mean age [15].
After 2 weeks of treatment, complete clinical cure was found in 23% patients in Group A and 19% patients in Group B. A study by Voravutinon also had similar result (19.4%) at the end of 2 weeks of treatment with terbinafine [15]. Similarly, Cochrane review on the topical antifungal treatments for tinea corporis suggested that treatment with terbinafine is effective with few adverse effects [16].
Improvement in clinical symptoms of tinea corporis was found in 85% of the patients on Terbinafine and Sertaconazole and 96% of the patients on Terbinafine and Amphotericin B in our study. A study by Cole et al also found similar improvement rates of 87% with 500 mg of oral Terbinafine for 6 weeks [17]. There was effective therapy in 77.4% at 2 weeks treatment and 87.1% at 6 weeks treatment with 250 mg oral terbinafine once daily. An effective therapy in 89.2% patients of tinea corporis was seen after 2 weeks of therapy with amphotericin B gel in a study by Sheikh et al. [9].
According to a study, 4 patients had mild adverse effects (nausea, diarrhea, and headache) but they subsided and all were able to complete their full course of treatment with Terbinafine [15]. Our study did not report any adverse effect. The reason for the difference could be due to the less dose and duration of the therapy. Moreover, in one of the groups, we used natural lipids as Amphotericin B gel, which reduced the chances of adverse reactions, making it compatible to the skin, and minimizing allergic reactions. Sheikh et al also did not report any adverse event in 83 patients of their study using lipid based amphotericin B gel [9].
We used 250 mg oral terbinafine with either Sertaconzole cream or Amphotericin B gel for 2 weeks treatment. It has been proved in various trials that the use of topical antifungals along with systemic antifungals has been found to be more useful in early achievement of clinical and mycological cure as well as decreasing the duration of oral antifungals leading to better patient compliance [4].
Comparing the skin related quality of life (DLQI) score; it was found that the mean in Group A and Group B was 7.38 and 7 respectively. A study by Hongbo reported the overall mean DLQI score of 4.86, which is comparable [14].
Various drawbacks of this study are as follows: the study could have a follow-up after 2 weeks to assess the complete cure of the patients of tinea corporis and to assess the adverse effects after 2 weeks after treatment with combination therapy. Also, the duration of treatment could also be increased to assess the efficacy of the treatment regime after 2 weeks of treatment.
Conclusion
The use of topical antifungals along with systemic antifungals has been found to be more useful in early achievement of clinical cure and improvement in clinical symptoms of tinea corporis. There is also decrease in the duration of oral antifungals, which leads to better patient compliance. More trials are needed to compare the various oral and topical antifungal therapies to give a clear idea regarding their dose and duration of the therapy. This study tried to bridge some gaps in the existing lacuna in the research on combination therapy of various topical and oral antifungal agents.
Source of Funding
This projects is a part of ICMR-STS (Indian Council of Medical Research – Short term Studentship Program) 2016. The project has been supported by ICMR-STS 2016 program.
-
- Havlickova B, Czaika VA, Friedrich M (2008) Epidemiological trends in skin mycoses worldwide. Mycoses. 51: 2-15 Link: https://goo.gl/Xgy6nw
- Likness LP (2011) Common dermatologic infections in athletes and return-to-play guidelines. J Am Osteopath Assoc 111: 373-379 Link: https://goo.gl/XoyKtL
- Akinboro AO, Olasode OA, Onayemi O, Mejiuini DA (2013) The impacts of Tinea capitis on quality of life: a community based cross sectionalstudy among Nigerian children. Clinical Medical Insights:Dermatology 6: 9-17 Link: https://goo.gl/ABjQpi
- Sahoo AK, Mahajan R (2016) Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J 7: 77-86 Link: https://goo.gl/UdbTNW
- Weinstein A, Berman B (2002) Topical Treatment of Common Superficial Tinea Infections. Am Fam Physician 65: 2095-102 Link: https://goo.gl/OckS4
- Rotta I, Otuki MF, Sanches ACC, Correr CJ (2012) Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras 58: 308-318 Link: https://goo.gl/zdck2s
- Choudhary SV, Bisati S, Singh AL, Koley S (2013) Efficacy and safety of terbinafine hydrochloride 1% cream vs. sertaconazole nitrate 2% cream in Tinea corporis and Tinea cruris: A comparative therapeutic trial. Indian J Dermatol 58: 457-460 Link: https://goo.gl/SKnN3C
- Shivamurthy RPM, Reddy SGH, Kallappa R, Somashekar SA, Patil D et al. (2014) Comparison of topical antifungal agent sertaconazole and clotrimazole in the treatment of Tinea corporis - An observational study. Journal of Clinical and Diagnostic Research 8: 9-12 Link: https://goo.gl/nzddVP
- Sheikh S, Ahmad A, Ali SM, Paithankar M, Barkate H et al. (2014) Topical delivery of lipid based amphotericin B gel in the treatment of fungal infection: a clinical efficacy, safety and tolerability study in patients. J Clin Exp Dermatol Res 5: 248 Link: https://goo.gl/tgh2tj
- Nigam PK (2015) Antifungal drugs and resistance: Current concepts. Our Dermatol Online 6: 212-221 Link: https://goo.gl/H6dWdq
- Callen P, Hughes AP, Kulp-Shorten C (2001) Subacute cutaneous lupus erythematosus induced or exacerbated by terbinafine: a report of 5cases. Arch Dermatol 137: 1196-1198. Link: https://goo.gl/Y4U86e
- Finlay AY, Khan GK (1994) Dermatology life quality index (DLQI): A simple practical measure for routine clinical use. Clin Exp Dermatol 19: 210-216 Link: https://goo.gl/tzEmQe
- Lewis VL, Finlay AY (2004) Ten years experience of the dermatology life quality index (DLQI). J Investig Dermatol Symp Proc 9: 169-180 Link: https://goo.gl/5qT55y
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY (2005) Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol 125: 659-664 Link: https://goo.gl/fVQu1n
- Voravutinon V (1993) Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: A randomized double blind comparative study. J Med Assoc Thai 76: 388-393. Link: https://goo.gl/mDVv2X
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, Burgess H, Doney L et.al. (2014) Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev 8: CD009992. Link: https://goo.gl/Q7xHCZ
- Cole GW, Stricklin G (1989) A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol 125: 1537-1539. Link: https://goo.gl/krexdx









Table 1:
Demographic profile of the patients.
Parameters
Group A (n=26)
Group B
(n=26)
p-value
Age (years) (Mean ± SD)
36.9±10.8
38.92±10.8
0.49
Sex (M:F)
13:13
10:16
0.41
Duration of disease (days) (Mean ± SD)
47.8±83.3
51.0 ±50.9
0.86
Family history of disease
5
7
0.52