Authors:
Faten LIMAIEM*, Saloua MEJRI, Imen KHELIL, Ahlem LAHMAR, Saadia BOURAOUI, Sabeh MZABI-REGAYA
Department of Pathology. Mongi Slim Hospital, La Marsa, University of Tunis El Manar, Tunis Faculty of Medicine, 1007, Tunisia
Received: 10 December, 2015; Accepted: 23 December, 2015;Published: 24 December, 2015
Faten Limaiem, Department of Pathology. Mongi Slim Hospital, La Marsa, University of Tunis El Manar, Tunis Faculty of Medicine, 1007. +216 96 55 20 57; E-mail:
LIMAIEM F, MEJRI S, KHELIL I, LAHMAR A, BOURAOUI S, et al. (2015) Adult Granulosa Cell Tumours of the Ovary Seven Case Reports. J Gynecol Res Obstet 1(1): 013-016.
© 2015 LIMAIEM F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Ovary; Adult granulosa cell tumour; Histopathology
Background: Ovarian granulosa cell tumours are low-grade malignant sex cord-stromal tumours. They represent 2 to 3% of all ovarian cancers and occur mainly within the adult population.
Aim: to provide an updated overview on ovarian adult granulosa cell tumours.
Patients and Methods: in our retrospective study, we reviewed seven cases of adult granulosa cell tumours that were diagnosed at the pathology department of Mongi Slim hospital over a fourteen-year period (2002- 2015). Clinical and pathRIiological characteristics were retrospectively analyzed.
Results: The patients of our series ranged in age between 39 and 64 years (mean = 53 years). The most common presenting symptom was abnormal uterine bleeding (n=5) followed by pelvic pain (n=4). All patients underwent surgical treatment including total hysterectomy with bilateral salpingo-oophorectomy (n=4), hysterectomy with right salpingo-oophorectomy (n=1) and salpingo-oophorectomy (n=2). Histopathological examination of the surgical specimen confirmed the diagnosis of adult granulosa cell tumour in all cases.
Conclusions: Adult granulosa cell tumours of the ovary are considered as low grade malignancies with a relatively more favourable prognosis compared with much more commonly encountered epithelial ovarian tumours. A prolonged post-therapeutic follow-up is necessary because of the risk of recurrences.
Introduction
Ovarian granulosa cell tumours (GCTs) are uncommon neoplasms that arise from the sex-cord stromal cells of the ovary and represent 2% to 3% of all ovarian cancers [1,2]. There are two histological forms: an adult form (95%) and a juvenile form (5%). Ovarian granulosa cell tumours are characterized by tendency to late recurrences and a favorable overall prognosis. In this paper, we report seven cases of adult GCTs of the ovary that were diagnosed at our institution over the past fourteen-year period. The aim of this study was to analyze epidemiological characteristics, clinical symptoms, radiological features, treatment and outcomes of seven patients who were surgically treated at our institution.
Patients and Methods
We undertook a retrospective study of seven patients who were operated on for ovarian adult GCTs at the Gynecology department of Mongi Slim hospital of Tunis between March 2002 and August 2015. The cases were retrieved from the files of the registry of surgery of the same hospital. Medical records were scrutinized for epidemiologic characteristics, predisposing factors, initial manifestations of the disease, methods of diagnosis, laboratory findings and surgical treatment. Diagnosis of the adult GCTs was based upon clinical, imaging and histopathological findings. All patients underwent imaging evaluation during the preoperative period. All specimens were surgically obtained. Tissues were fixed in 10% phosphate buffered formaldehyde, embedded in paraffin and sections were prepared for routine light microscopy after staining with hematoxylin and eosin. Patient confidentiality was maintained.
Results
Clinical findings
Our study group included seven female patients between 39 and 64 years of age (mean = 53 years). Two patients presented with co-morbidities namely hypertension (n=1) and diabetes (n=1). The presenting clinical symptoms were dominated by abnormal uterine bleeding (n=5) followed by pelvic pain (n=4) (Table 1).
Radiological findings and localization
Diagnostic imaging techniques included ultrasonography in all cases and CT scan in two cases. In our series, five tumours were heterogeneously solid and two were multiseptated cystic masses. Hemorrhage was present in two cases.
Treatment
All patients underwent surgical treatment including total hysterectomy with bilateral salpingo-oophorectomy (n=4), hysterectomy with right salpingo-oophorectomy (n=1), and salpingo-oophorectomy (n=2).
Pathologic findings
Macroscopic findings (Figures 1,2): In our series, ovarian adult GCTs varied in size from 6,5 to 23 cm (mean = 15,14 cm). On cut section, the tumours were solid and cystic. The solid areas were soft and tan to yellow. Necrosis was noted in four cases and hemorrhage in two cases. Histopathological examination of the surgical specimen (Figures 3,4) confirmed the diagnosis of adult GCTs in all cases. In one case, endometrial hyperplasia was noted. Histopathological findings of ovarian adult GCTs of our series are summarized in Table 2.
-
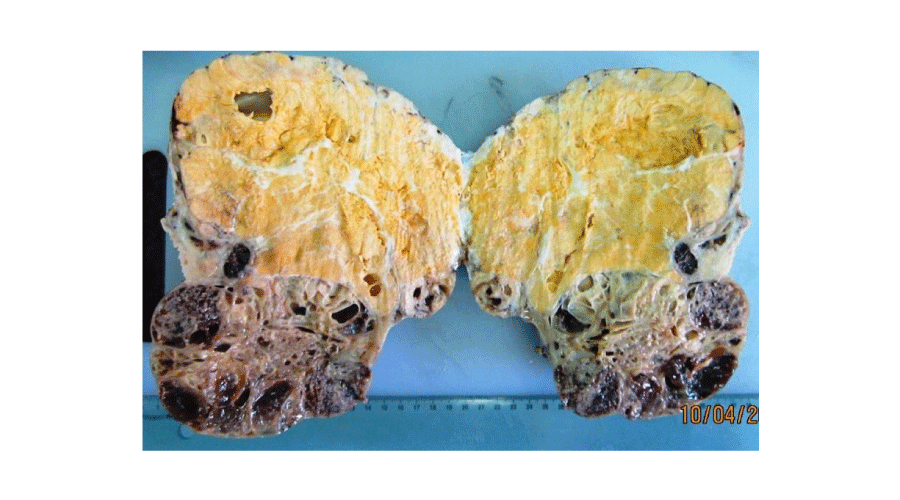
Figure 1:
Macroscopic findings of adult granulosa cell tumour. Solid yellow-brown mass with small cysts.
-
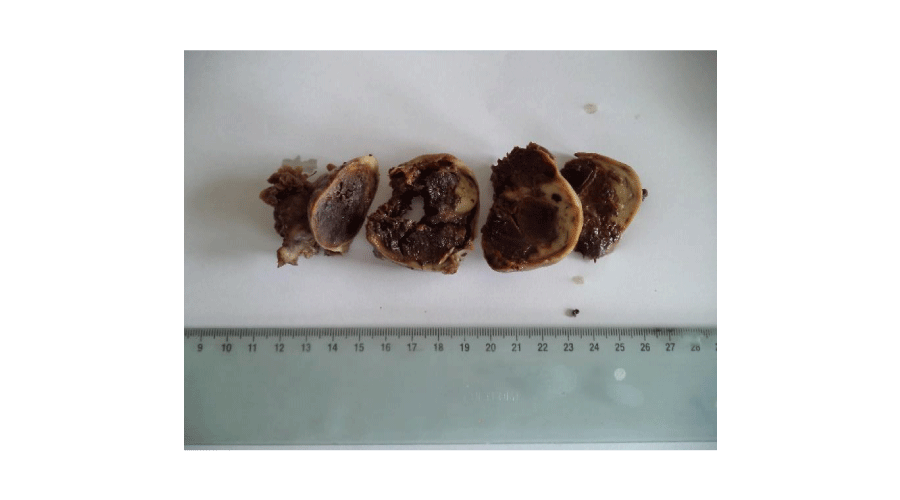
Figure 2:
Macroscopic findings of adult granulosa cell tumour. Solid brown mass with foci of haemorrhage.
-
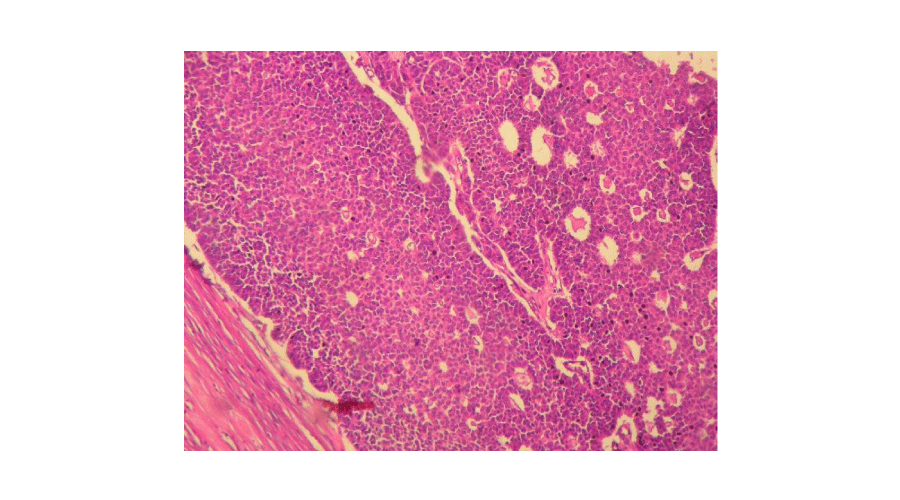
Figure 3:
Tumour cells showed a diffuse and microfollicular pattern (Hematoxylin and eosin, magnification × 200).
-
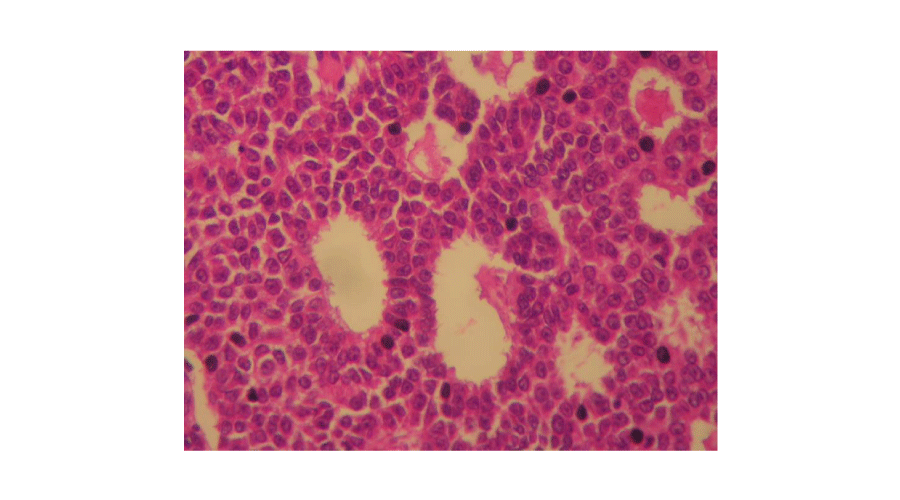
Figure 4:
Microfollicular pattern (Call-Exner bodies). The tumour cells with occasional grooved nuclei surrounded small rounded spaces filled with eosinophilic material (Hematoxylin and eosin, magnification × 400).
-

Table 2:
Histological findings of ovarian adult granulosa cell tumours in our series.
Follow-up and evolution
Postoperative course was uneventful in all cases. The follow-up period ranged between two months and two years. Three patients were lost to follow-up. The other patients are still being followed-up.
Discussion
Adult GCTs are low-grade malignant, sex cord stromal tumours composed of granulosa cells often with a variable number of fibroblasts and theca cells [3]. They occur in the peri- and postmenopausal period with a peak prevalence in patients aged 50 to 55 years. The other peak frequency corresponds to the prepubertal age [4,5]. The symptoms are various: abdominal pain (30 to 50%), abdominal distension related to mass effect and hormonal events (41%) such as irregular menstruation, intermenstrual bleeding, postmenopausal bleeding or amenorrhea [6]. Endocrine manifestations are noted in 66% of the patients. These manifestations are related to estrogen secretion of the tumour [7]. This explains why the adult GCTs are frequently associated with endometrial hyperplasia (4 to 10%) or to endometrial adenocarcinoma (5 to 35%) [7,8]. Imaging findings in adult GCTs vary widely and range from solid masses, to tumours with varying degrees of hemorrhagic or fibrotic changes, to multilocular cystic lesions to completely cystic tumours. Ko et al., categorized 13 adult GCTs into 5 morphologic patterns based on ultrasonographic and CT scan findings: multilocular cystic, thick-walled unilocular cystic, thin-walled unilocular cystic, homogenously solid, and heterogeneously solid. Intratumoral bleeding, infarcts, fibrous degeneration and irregularly arranged tumour cells have yielded heterogeneously solid tumours [9,10]. Evidence of hemorrhage has been reported in 60-71.4% of cases [10,11]. In contrast with epithelial neoplasms, GCTs do not have intracystic papillary projections, less propensity for peritoneal seeding, and are confined to the ovary at the time of diagnosis. Therefore, endometrial and cervical biopsies are essential to define the therapeutic strategy. Grossly, adult GCTs vary greatly in size, but the average diameter is about 10 cm. In our series, adult GCTs varied in size from 6,5 to 23 cm (mean = 15,14 cm). They are most typically solid and cystic, but may be solid or rarely entirely cystic. The solid areas are usually soft and tan to yellow. The cysts typically contain clotted blood and some tumours particularly those associated with rupture, exhibit conspicuous haemorrhage [3]. Histologically, a variety of growth patterns occur and are often admixed. The most common pattern is diffuse, in which the tumour cells grow in sheets. Tumour cells often grow in cords and trabeculae, in undulating ribbons and in nests (insular pattern). A microfollicular pattern (Call-Exner bodies), in which granulosa cells surround small spaces containing eosinophilic secretion, sometimes with nuclear debris or occasionally hyaline material, is seen in a minority of tumours and is uncommonly conspicuous [3]. Occasionally larger follicles are seen (macrofollicular pattern). A pseudopapillary architecture may be seen. The tumour cells usually have scant pale cytoplasm. The nuclei are typically uniform, pale and round to oval. Nuclear grooves are a characteristic feature but in many tumours are not conspicuous. Nuclear atypia is usually absent except for occasional cases (about 2%) which show bizarre nuclei. Mitotic activity is variable and sometimes brisk. Granulosa cell tumours contain a variable amount of fibromatous or thecomatous stroma [3]. Immunohistochemically, GCTs usually exhibit inhibin, calretinin, FOXL2, steroidogenic factor-1 (SF-1), WT1 and CD56 positivity [3]. They may be positive for broad spectrum and low molecular weight (8 and 18) keratins but are typically negative for CK7 and EMA. They may be positive for smooth muscle actin, desmin, CD99 and S-100 protein [12,13]. The most common abnormalities reported have been trisomy 12, trisomy 14, monosomy 16 or deletion of 16q and monosomy 22. There is a missense somatic point mutation in the FOXL2 gene (402 C to G) in more than 90% of adult GCTs [13]. Surgery is advocated as the first treatment of choice, because it provides the accurate information about the initial extent of disease, and therefore it documents the patients requiring adjuvant treatment modalities. Although the extent of the initial surgical procedure is still controversial and not standard, some authors reported higher relapse rates in cases of conservative surgery and better survival in cases with radical surgery. In patients of childbearing age with desire for future fertility, fertility saving surgery seems to be acceptable [14]. Granulosa cell tumours of the ovary are considered as low grade malignancies with a relatively more favorable prognosis compared with much more commonly encountered epithelial ovarian tumors. However, patients diagnosed with GCT still suffer from recurrence or disease-related mortality necessitating surgery and/or other treatment modalities. In most studies, disease stage, patient’s age and presence or absence of residual disease after initial surgery were shown to be important prognostic factors in GCTs [6,15]. The recurrence rate of adult GCTs is 10-15% for stage Ia tumours and 20-30% overall. Metastases or recurrences are often detected more than five years after initial treatment, sometimes after intervals > 20 years [6,15-18]. Extra-ovarian spread is to the peritoneum and omentum and rarely to liver or lungs. Lymph node metastases are uncommon. Unfavourable factors include advanced stage, large size (>15 cm), bilaterality and tumour rupture. There is no correlation between microscopic appearance and prognosis, including mitotic activity and outcome [12].
In summary, this retrospective study from Tunisia provides an overview on clinicopathological features in seven patients with ovarian adult GCTs. Granulosa cell tumours of the ovary are considered as low grade malignancies with a relatively favourable prognosis. Accurate diagnosis and staging of these tumours is critical for optimal treatment planning and for determining prognosis. A prolonged post-therapeutic follow-up is necessary because of the risk of recurrences.
-
-
-
- Schumer ST, Cannistra SA (2003) Granulosa cell tumor of the ovary. J Clin Oncol 21: 1180–1189.
- Unkila-Kallio L, Tiitinen A, Wahlstrom T, Lehtovirta P, Leminen A (2000) Reproductive features in women developing ovarian granulosa cell tumour at a fertile age. Hum Reprod 15: 589–593.
- Zaloudek CJ, Mooney EE, Staats PN, Young RH (2014) Sex cord-stromal tumours - pure sex cord tumours. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH dir. WHO classification of tumours of Female reproductive organs. Lyon: Int Agency Res Cancer 50-51.
- Fujimoto T, Sakuragi N, Okuyama K, Fujino T, Yamashita K, et al. (2001) Histopathological prognostic factors of adult granulosa cell tumors of the ovary. Acta Obstet Gynecol Scand 80: 1069–1074.
- Stenwig JT, Hazekamp JT, Beecham JB (1979) Granulosa cell tumors of the ovary: clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol 7: 136–152.
- Ayhan A, Salman MC, Velipasaoglu M, Sakinci M, Yuce K (2009) Prognostic factors in adult granulosa cell tumors of the ovary: a retrospective analysis of 80 cases. J Gynecol Oncol 20: 158-163.
- Bompas E, Freyer G, Vitrey D, Trillet-Lenoir V (2000) Granulosa cell tumour: review of the literature. Bull Cancer 87: 709–714.
- Segal R, DePetrillo AD, Thomas G (1995) Clinical review of adult granulosa cell tumors of the ovary. Gynecol Oncol 56: 338–344.
- Ko SF, Wan YL, Ng SH, Lee TY, Lin JW, et al. (1999) Adult ovarian granulosa cell tumors: spectrum of sonographic and CT findings with pathologic correlation. AJR Am J Roentgenol 172: 1227–1233.
- Morikawa K, Hatabu H, Togashi K, Kataoka ML, Mori T, et al. (1997) Granulosa cell tumor of the ovary: MR findings. J Comput Assist Tomogr 21: 1001-1004.
- Kim SH, Kim SH (2002) Granulosa cell tumor of the ovary: common findings and unusual appearances on CT and MR. J Comput Assist Tomogr 26: 756-761.
- Cathro HP, Stoler MH (2005) The utility of calretinin, inhibin and WT1 immunohistochemical staining in the differential diagnosis of ovarian tumours. Hum Pathol 36: 195-201.
- Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, et al. (2009) Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. Am J Surg Pathol 33: 354-366.
- Pautier P, Lhomme C, Culine S, Duvillard P, Michel G, et al. (1997) Adult granulosa-cell tumor of the ovary: a retrospective study of 45 cases. Int J Gynecol Cancer 7: 58-65.
- Malmstrom H, Hogberg T, Risberg B, Simonsen E (1994) Granulosacell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol 52: 50-55.
- Fugimoto T, Sakuragi N, Okuyama K, Fujino T, Yamashita K, et al. (2001) Histopathological prognostic factors of adult granulosa cell tumors of the ovary. Acta Obstet Gynecol Scand 80: 1069-1074.
- Hines JF, Khalifa MA, Moore JL, Fine KP, Lage JM, et al. (1996) Recurrent granulosa cell tumor of the ovary 37 years after initial diagnosis: a case report and review of the literature. Gynecol Oncol 60: 484-488.
- Schumer ST, Cannistra SA (2003) Granulosa cell tumor of the ovary. J Clin Oncol 21: 1180-1189.
-











Table 1:
Clinicopathological findings of adult granulosa cell tumours of the ovary of our series.
bleeding
Pelvic mass
No recurrence
Follow-up = two years
No recurrence
Follow-up = 14 months
Intermenstrual
bleeding
No recurrence
Follow-up = 12 months
Bleeding
Pelvic pain
No recurrence
Follow-up = 2 months