Author(s):
Amit Arvind Agrawal1*, Swapnil Kolhe2, Amit Sope3 and Dinesh Erlewad4
1Department of Periodontics and Implantology, MGV’s KBH Dental College and Hospital, Nasik, Maharashtra, India
2Department of Conservative Dentistry and Endodontics, MGV’s KBH Dental College and Hospital, Nasik, Maharashtra, India
3Department of Cell Immunology and Biology, University of North Texas Health Science Center, Texas, USA
4Principle Dentist, 208, Sterling Center, M.G road, Camp, Pune 411001, Maharashtra, India
Received: 03 May, 2016; Accepted: 11 May, 2016; Published: 13 May, 2016
Dr. Amit Arvind Agrawal, BDS, MDS, MPhil, Professor, Department of Periodontics and Implantology, MGV’s KBH Dental College and Hospital, Mumbai-Agra road, Panchvati, Nashik-422003, Maharashtra, India, Tel: +919822107562; E-mail:
Agrawal AA, Kolhe S, Sope A, Erlewad D (2016) Root Canal Disinfection Potential of 5.25% Sodium Hypochlorite, 2% Chlorhexidine and 810nm Diode Laser-A Comparative In vitro Antimicrobial Study. Int J Oral Craniofac Sci 2(1): 035-038. 10.17352/2455-4634.000016
© 2016 Agrawal AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Root canal disinfection; Sodium hypochlorite; Chlorhexidine; Diode laser; Enterococcus fecalis
E.faecalis: Enterococcus faecalis; Nacl: Sodium Chloride; DNA: Deoxyribonucleic Acid; RNA: Ribonucleic Acid; NaOCl: Sodium Hypochlorite; DTA: Ethylene Diamino Tetra Acetic Acid; CHX: Chlorhexidine; DL: Diode Laser; CB group: Control-Baseline Group; C BMP group: ‘Control-Biomechanical Preperation’ Group; NCIM: National Collection of Industrial Micro-organisms; ATCC: American Type Culture Collection; BMP: Bio-Mechanical-Preperation; LASER: Light Amplification of Stimulated Emission of Radiation; Nd: YAG: Neodymium-doped Yttrium Aluminum Garnet; W: Watts
Background: The eradication of persisting bacteria, such as Enterococcus faecalis, is crucial for the long-term preservation of the endodontically treated tooth.
Context and Purpose of the study: The aim of this research was to evaluate and compare the root canal disinfection potential of 5.25% sodium hypochlorite, 2% chlorhexidine gluconate and 810nm diode laser against control.The aim of this research was to evaluate and compare the root canal disinfection potential of 5.25% sodium hypochlorite, 2% chlorhexidine gluconate and 810nm diode laser against control.
Results: Adjunctive use of chemical disinfection by either 5.25% sodium hypochlorite or 2% chlorhexidine led to 100% microbial eradication as against diode laser which achieved 97.7% reduction as compared to baseline microbial count and 68.42% reduction after mechanical cleaning at the same dilutions.
Main findings: Chemicals used in the study achieved greater disinfection than diode laser irradiation.
Conclusions: 5.25% sodium hypochlorite or 2% chlorhexidine can be efficiently used as an adjuvant to mechanical root canal cleaning.
Brief summary: A total of 20 extracted teeth, sectioned at cement-enamel junction, were divided into four groups of five teeth each. Control group: mechanical cleaning only; three test groups: mechanical cleaning followed by disinfection with 5.25% sodium hypochlorite or 2% chlorhexidine or 810nm diode laser. Pre and Post treatment microbial samples were collected and cultured.
Potential Implications: A thorough mechanical instrumentation is crucial for success of any endodontic therapy and chemical or laser irradiation will only be helpful as an adjuvant.
Introduction
The major cause of endodontic failure is the survival of microorganisms in the apical portion of root filled teeth, of which, E.faecalis is considered one of the primary organisms in patients with post treatment endodontic infection [1]. Enterococci were first placed under genus streptococcus, however studies demonstrated a more distant relationship with streptococci [2]. In 1984, enterococci were given a formal genus status after DNA-DNA and DNA-RNA hybridization. They are gram positive facultative anaerobic coccoid bacteria which can occur singly, in pairs or as short chains. Enterococci grow at temperatures ranging from 10-450C, at pH 9.6 and in 6.5% (NaCl) sodium chloride and can survive at 600C for 30 minutes. E.faecalis in particular, possesses certain virulence factors including lytic enzymes, aggregation substance, pheromones and lipoteichoic acid [3]. E.faecalis has the ability to establish monoinfections in medicated root canals. The organism has the ability to acquire, accumulate and share extra-chromosomal elements, encoding virulence traits, which help to colonize, compete with other bacteria, resist host defense mechanisms and produce pathological changes directly through the production of toxins or indirectly through the induction of inflammation. E.faecalis has the advantage of forming biofilms, hence it has a certain degree of protection and homeostasis. Biofilms grow in a nutrient-deprived ecosystem as it concentrates trace elements and nutrients by physical trapping and electrostatic interaction. The bacterial cells residing in a biofilm communicate, exchange genetic materials and acquire new traits. This communication takes place by quorum sensing. E.faecalis is also known to resist intra canal medicaments like calcium hydroxide by maintaining pH homeostasis [4].
An in-vitro study by Hohscheidt et al5 to evaluate the effect of different endodontic auxillary chemical substance such as (NaOCl) sodium hypochlorite, EDTA (ethylene diamino tetra acetic acid), 2%CHX (chlorhexidine) gel, 2% CHX liquid in different combinations, concluded that none of the tested substances could completely eliminate the E.fecalis from the root canal space. In addition, few in-vitro studies [6-8], have evaluated the disinfection potential of diode laser following chemo-mechanical procedures against E.fecalis, and concluded that 980nm diode laser can even eliminate bacteria that has immigrated into dentin, thus being able to increase the success rate in endodontic therapy. To our knowledge there is no study which has evaluated the disinfection potential of sodium hypochlorite, chlorhexidine and 810nm diode laser together.
The aim of this in-vitro microbial research was to evaluate and compare the root canal disinfection potential of 5.25% NaOCl, 2% CHX and 810nm diode laser (DL) against control (C). Our baseline null hypothesis was that 5.25%NaOCl, 2% CHX and 810nm diode laser are equally effective in eradication of E.fecalis from root canal space in vitro.
Materials and Methods
Twenty extracted single rooted teeth were sectioned at the cemento-enamel junction and the roots were prepared by step back technique to #30 K- file (Maillefer, Dentsply) at the apical end. All the teeth were then sterilized by autoclaving. The root canal spaces were then filled with liquid MRS medium containing pure culture strains of E.fecalis* (NCIM no. 5024) (ATCC no. 14506) and inoculated for 24 hrs in an incubator. E.fecalis culture was obtained from National Collection of Industrial Micro-organisms (NCIM), National Chemical Laboratory (NCL), Pune-411008, India.
Twenty samples were divided into 4 groups of 5 teeth each, the groups were as follows:
a. Control group:
i. Control Baseline (CB group) – 2 teeth.
ii. Control BMP, (C BMP group) – 3 teeth.
b. 2% Chlorhexidine group (CHX)
c. 5.25% sodium hypochlorite group (NaOCl)
d. Diode LASER group (Laser)
All the sample teeth along with the experimental materials were kept in the laminar air flow, and following techniques were performed depending on the study group.
Control group
After 24 hrs of inoculation, verification of count of bacteria inoculated in root canal was done with 2 samples (CB group), out of total 5 samples in control group, which displayed innumerable/uncountable colonies in MRS medium. The inner wall of remaining 3 teeth (C BMP group), were cleaned mechanically (for 1 min) using K files (Maillefer, Dentsply), followed by sterile saline irrigation (10ml) using 30 guage Max I probe needle.
2% Chlorhexidine (CHX) group
Mechanical cleaning with K files (Maillefer, Dentsply), was done for 1 min by brushing technique, followed by 5ml irrigation of 2% chlorhexidine (Dentochlor, Ammdent, Amrit Chemical, Mohali, India) for 30 seconds using 30 gauge Max I probe needle; followed by sterile saline irrigation (10ml).
5.25% sodium hypochlorite (NaOCl) group
Mechanical cleaning with K files (Maillefer, Dentsply), was done for 1 min by brushing technique, followed by 5ml irrigation of 5.25% sodium hypochlorite solution (Prime Dental Products, Mumbai, India) for 30 seconds using 30 guage Max I probe needle, followed by sterile saline irrigation (10ml).
Diode LASER (Laser) group
Mechanical cleaning with K files (Maillefer, Dentsply) was done for 1 min, followed by irradiation with a diode LASER (Picasso, Dentsply) in non-contact mode, continuous wave, 3W setting. A single cycle consisted of an exposure for 5sec and a rest of 15 sec, total 5 such cycles were completed for each of the 5 samples in this group. The fiber from the laser hand piece (tip diameter 30 microns) was introduced into the root canal up to the apex and then the laser is activated. The fiber was guided in an apical to coronal direction with circular movements. Finally irrigation was done using 10ml of sterile saline.
After these disinfection stages, three paper points (#20, Maillefer, Dentsply) were inserted, one by one, in the canal of each of 20 sample teeth and then transferred to a test tube containing 1ml of peptone water. The test tube was vortexed to dislodge any microbial colonies attached to the paper point. 1 ml of this peptone water is diluted with 9ml of sterile saline; 1ml from the resultant 1:10 dilution of bacteria is then mixed with 15ml of MRS medium using pour plating method and inoculated for 48 hrs.
Furthermore, 1ml of the 1:10 dilution solution is again diluted with 9 ml of sterile saline; 1ml from the resultant 1:100 dilution of bacteria is then mixed with 15ml of MRS medium using pour plating method and inoculated for 48 hrs.
Still further, 1ml of the 1:100 dilution is diluted with 9 ml of sterile saline; 1ml from the resultant 1:1000 dilution of bacteria is then mixed with 15ml of MRS medium using pour plating method and inoculated for 48 hrs. However, this 1:1000 dilution was required only in control group samples, where in the initial dilution, innumerable colonies were obtained.
Results
After 48 hrs, all petri-dishes were recovered from the inoculation chamber and the colonies were physically counted. Only 2 plates had such innumerable count that it could have been impossible to get an exact count. In these samples a higher dilution (1:1000) count was considered.
Table 1 shows the number of colonies in each group and at various dilutions. At 1:10 dilution, the baseline microbial count in untreated samples was uncountable but after biomechanical preparation (BMP) the count reduced to an average of 30.5 colonies. At 1:100 dilution, this ratio was 66.33 to 4.75, (Figure 1a,b) that means there was about 92.83% reduction in the microbial count simply by thorough mechanical cleaning. This finding further strengthens the fact that all disinfecting agents can be an adjuvant to mechanical cleaning and never a replacement. In two test groups, that using chlorhexidine and sodium hypochlorite irrigation, the colony counts were zero at 1:10 or 1:100 dilutions (Figure 1c,d). However in the diode laser group, an average of 1.5 colonies were noted at 1:100 dilution (Figure 1e), which is 97.73% reduction as compared to baseline microbial count and 68.42% reduction after mechanical cleaning at the same dilutions.
-
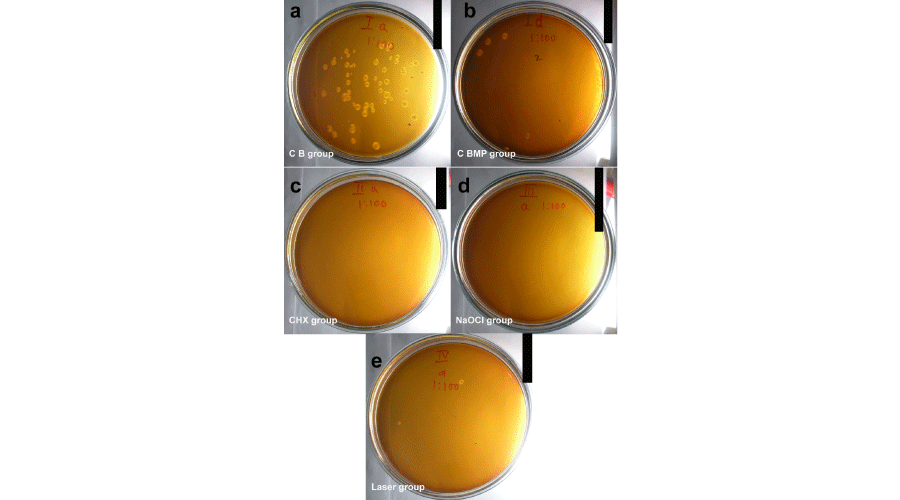
Figure 1:
Shows number of colonies grown on 1:100 dilution MRS medium in different study groups. a: control baseline group (without BMP); b: control group after BMP; c: Chlorhexidine group; d: Sodium hypochlorite group; e: diode laser group.
Discussion
The primary aim of disinfection of the root canals intentionally inoculated with E.faecalis bacteria was successfully achieved in the present study. Pre-disinfection colony count was innumerable; convincing that disinfection is surely required. As mechanical means are considered the goal standard, BMP did reduce the colony counts from an average of 66.33 to 4.75 (92.83%) (at 1:100 dilution), but it was still not a complete eradication. These findings are consistent with an in-vitro study by Machado et al. [5], where they found that bacterial reduction was 81.94% and 84.29% after root canal preparation by two different rotary instruments. As compared to Machado et al study, we have found greater reduction in bacterial count after BMP, which could be because molar teeth were used in their study and we have used anterior single rooted teeth only. However this finding supports that a thorough BMP is key to a long term successful endodontic therapy. The other advantage of a good BMP is that it would allow access to additional means of disinfection to reach the apical third of canal which otherwise would not have been possible.
Taking this into account, BMP was performed in all samples before evaluating the effect of adjunctive disinfection agent. Sodium hypochlorite is been used in clinical practice since a long time and reports of chemical irritation to peri-apical areas and/or surrounding gingival tissue are also often encountered. However, literature does support the use 5.25% sodium hypochlorite as a potentially safe and effective disinfection agent. Chlorhexidine 2% involves the advantage over NaOCl with regard to its tissue irritating property. Maria Teresa et al. [9], observed in their studies that it was not possible to eradicate E.faecalis biofilms using chlorhexidine alone. They found that the alternating use of chlorhexidine and cetrimide (0.1% and 0.05%) killed 100% of E.faecalis biofilm cultures. However, in the present study, we could demonstrate 100% elimination of bacteria after BMP followed by irrigation with either 2%CHX or 5.25%NaOCl. Whether both these chemicals could prove to be equally effective without doing BMP is not been addressed.
Adjunctive disinfection by diode LASER helped in reducing the bacterial colonies from an average of 4.75 after BMP alone to 1.5 after BMP and laser (at 1:100 dilution), however it was still not a complete eradication. The primary use of lasers in endodontics is focused on eradicating micro-organisms in the root canal, especially in the lateral dentinal tubule. This requires a wavelength that shows high transmission through hydroxyapatite and water. Absorption curves show that Nd:YAG Neodymium-doped Yttrium Aluminum Garnet) lasers, and in particular pulsed Nd:YAG lasers, are first-choice for this application. Nd:YAG lasers show the best results in transmission and micro-organism reduction measurements. Even at penetration depths exceeding 1000 μm, 85 % reduction is achieved. The 810 nm diode laser is the second-choice laser source. However diode laser are the most widely used laser among general practitioner worldwide due to its comparatively low cost in comparison to Nd:YAG and also may be due to its portability and wide range of applications. Hence diode laser was evaluated in this study inspite of the fact that Nd:YAG are considered first choice.
Microbiological studies have shown that this source provides the second highest micro-organism reduction, approximately 63%. This is nevertheless significantly lower than with Nd:YAG lasers. Diode lasers (980 nm) may also be an option, although high transmission is achieved due to its higher absorption in water. This explains why this laser source, especially at a depth of 1000μm, can only achieve 30% to 40% micro-organism reduction. Gunwal et al. [10], showed that 810nm diode laser reduces microbial count more significantly as compared to 5.25% NaOCl, 2% Chlorhexidine and MTAD solution.
Our results were consistent with the findings of Mashalkar et al. [11], who concluded from their in-vivo comparative study that conventional method of root canal disinfection using sodium hypochlorite and hydrogen peroxide as irrigating solutions were highly effective, however lasers when used can also reduce the bacterial load of the infected root canal. Few studies are supporting our results [11-14].
Paper point cultures of the root canal detected bacteria more frequently than dentine filling cultures on the reamers [11], and hence it was the preferred mode of sample collection throughout the study. Pour plating method involves spreading the sample in the petri dish first, followed by pouring of medium and mixing them both. This technique is considered to give better readings in terms of colony counting as compared to the surface plating method. Since, pour plating is technique sensitive, this section of the study was performed by an experienced microbiologist.
To conclude, BMP is the basic and most important step in our progress towards achieving 100% disinfection of root canals, in terms of mechanically removing the micro-organisms and allowing effective use of adjunctive disinfection mediums. Within the scope of this research we found that chemical disinfection with either 2% CHX or 5.25%NaOCl are helpful in achieving complete eradication of E.fecalis from the root canals, whereas diode LASER was partially effective.
Acknowledgement
All equipments, culture media and other laboratory instruments for microbial analysis was provided by ‘BAC-TEST laboratory’ under supervision of microbiologist Mrs. Smita Khedkar.
Limitations
1. Although previous study [8], have shown that the diode laser parameters that induce cavitation do not result in adverse thermal changes in radicular dentin, the amount of heat generated and/or accumulated in the tooth or surrounding tissues was not evaluated in the present study.
2. Only single rooted teeth were analyzed, the results obtained could vary when the same procedures are followed in multi rooted teeth.
3. Diode lasers at different energy settings and treatment cycles may have a better or poorer outcome as obtained in the present study.
4. Since Nd:YAG laser was not available, we could not validate its potential in root canal disinfection. Future studies should strongly consider its use in their research.
- Shrestha A, Shi Z, Neoh KG, Kishen A (2010) Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod 36: 1030-1035.
- Portenier I, Waltimo TMT, Haapasalo M (2003) Enterococcus faecalis-the root canal survivor and ‘star’ in post treatment disease. Endodontic Topics 6: 135-159.
- Stuart CH, Schwartz SA, Beeson TJ, Owatz CB (2006) Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod 32: 93-98.
- Evans M, Davies JK, Sunqvist G, Figdor D (2002) Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J 35: 221-228.
- Hohscheidt GL, Böttcher DE, Fatturi Parolo CC, Montagner F, Grecca FS (2013) Response of E. faecalis biofilms to different associations of auxiliary substances during root canal preparation: a confocal laser microscopy analysis. Microsc Res Tech 76: 658-662.
- Kaiwar A, Usha HL, Meena N, Ashwini P, Murthy CS (2013) The efficiency of root canal disinfection using a diode laser: in vitro study. Ind J Dent Res 24: 14-18.
- Hmud R, Kahler WA, Walsh LJ (2010) Temperature changes accompanying near infrared diode laser endodontic treatment of wet canals. J Endod 36: 908-911.
- Machado ME, Sapia LA, Cai S, Martins GH, Nabeshima CK (2010) Comparison of two rotary systems in root canal preparation regarding disinfection. J Endod 36: 1238-1240.
- Arias-Moliz MT, Ferrer-Luque CM, Gonzalez-Rodriguez MP, Valderaama MJ, et al. (2010) Eradication of Enterococcus faecalis biofilms by cetrimide and Chlorhexidine. J Endod 36: 87- 90.
- Gunwal M, Shenoi P (2013) Evaluation of the efficacy of 5.25% of sodium hypochlorite, 2% of chlorhexidine, mtad and 810 diode laser in reduction of microbial count in root canal - an in vivo study. Endodontol 25: 56-62.
- Mashalkar S, Pawar MG, Kolhe S, Jain DT (2014) Comparative evaluation of root canal disinfection by conventional method and laser: an in vivo study. Niger J Clin Pract 17: 67-74.
- Luddin N, Ahmed HM (2013) The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent 16: 9-16.
- Gerek M, Asci S, Yaylali DI (2010) Ex-vivo evaluation of antibacterial effects of Nd:YAG and diode lasers in root canals. Biotechnol & Biotechnol eq 24: 2031-2034.
- Ashofteh K, Sohrabi K, Iranparvar K, Chiniforush N (2014) In vitro comparison of the antibacterial effect of three intracanal irrigants and diode laser on root canals infected with Enterococcus faecalis. Iran J Microbiol 6: 26-30.









Table 1:
Shows the number of colonies (individual teeth and average) in each group and at various dilutions.
CB: control group baseline counts; C BMP group: Control group colony counts after BMP; CHX group: 2% chlorhexidine irrigation; NaOCl group: 5.25% sodium hypochlorite irrigation; Laser group: 810nm diode laser group.