Authors:
Prampart A1*, Djennaoui I1, Ciftci S1, Riehm S2 and Debry C1
1Department of Otorhinolaryngology (ENT) and Head and Neck Surgery, Strasbourg University Hospital, CHRU Hautepierre, 1 Avenue Molière 67100 Strasbourg, France
2Department of Radiology, Strasbourg University Hospital, CHRU Hautepierre, 1 Avenue Molière 67100 Strasbourg, France
Received: 28 April, 2017; Accepted: 27 May, 2017; Published: 29 May, 2017
Alexandre Prampart, Service ORL & Chirurgie cervico-faciale, Hôpitaux Universitaires de Strasbourg, CHRU Hautepierre, 1 Avenue Molière 67100 Strasbourg, France, E-mail:
Prampart A, Djennaoui I, Ciftci S, Riehm S, Debry C (2017) Postoperative Correlation of Radiological and Surgical Findings in Management of Ethmoid Sinus Adenocarcinoma. Arch Otolaryngol Rhinol 3(2): 051-55. DOI: 10.17352/2455-1759.000045
© 2017 Prampart A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Adenocarcinoma; Ethmoid; MRI; Monitoring; Recurrence
Aims:Prognosis of ethmoid sinus adenocarcinoma (ADK) is essentially determined by local tumor control. There is a high rate of recurrence of these tumors across the range of patient series. Development of an optimal follow-up protocol of such tumors is recommended.
Patients and methods:A retrospective, monocentric study was carried out including all patients diagnosed with ADK who underwent surgery and were followed up at our center between 2012 and 2016 and who were monitored postoperatively using magnetic resonance imaging (MRI) and histopathological verification of suspicious areas identified via imaging. Time to postoperative MRI, time to recurrence and sites of recurrence were obtained for each patient.
Objectives:Performance evaluation of MRI in early screening of recurrence or residualtumors postoperatively in the management of ADK and identification of the main sites prone to risk of recurrence in these tumors.
Results:We included 24 cases of ADK, there were 33% cases of recurrence with a mean time to recurrence of 35 months postoperatively. Mean time to completion of the first MRI scan was 65 days postoperatively.
Performance parameters of screening for recurrence or residual tumors on the first postoperative MRI were:
Se 64%; Sp 78%; PPV 69%; NPV 74%.
Conclusions: Efficacy of postoperative MRI screening appears to be limited and regular endoscopic monitoring associated with imaging is required. Sites prone to risk should be subject to particular consideration in primary surgical resection and management of recurrence.
The benefit of imaging in the immediate postoperative period has yet to be assessed in terms of disease-free survival and disease control.
Introduction
Paranasal sinus and nasal fossa cancers are rare tumors which are found most commonly in the ethmoid sinuses (5 % - 30%) [1,2]. Adenocarcinoma of the ethmoid sinus (ADK) is the prevailing histological type and originates in the olfactory cleft [1-4]. Prognosis of this type of neoplasia is essentially determined by initial local control of the disease.
Current optimal treatment of ethmoid sinus tumors is based on an association of complete surgical resection and adjuvant radiotherapy [5-10].
Despite recent innovations in such treatment (quality of endoscopic optics, endonasal instrumentation, intensity-modulated conformal radiotherapy…) clinical evolution nevertheless demonstrates a high rate of locoregional recurrence (30% of cases on average) involving complications principally due to complex anatomy and proximity to important anatomic structures [11-13].
Tumors are predominantly monitored using magnetic resonance imaging (MRI) and endoscopic examination under local anesthetic by appointment and/or general anesthetic involving biopsy in the event of the slightest doubt.
This study aims i) to evaluate the benefit of the first postoperative MRI in detecting tumor recurrence ii) to determine its benefit in surgical management of recurrence iii) to identify the main sites prone to risk of recurrence.
Patients and Methods
We carried out a retrospective monocentric study on 24 patients (n=24) treated in our center for primitive ADK during the period from 2012 to 2016. Inclusion criteria comprised all cases of initial or recurrent primitive ADK having undergone surgical resection followed by MRI monitoring (indifferently 1,5 or 3 Tesla, depending on the availability of the devices on the date of follow-up) and histological confirmation of suspicious areas detected on imaging.
We excluded
i) all primitive ethmoid sinus tumors which were not adenocarcinomas
ii) tumors which did not receive postoperative MRI monitoring.
For each patient we recorded the gender, age, incidence of occupational exposure to risk, initial loco-regional extension of the tumor shown on imaging, initial evidence of lymphadenopathy, TNM stage, initial treatment, selection of surgical approach (endonasal or external), presence of treatment adjuvant to surgery, delivered dose and delivery duration involving external radiotherapy, type of chemotherapy, period of time before completion of postoperative MRI, incidence of recurrence including site of recurrence and time to recurrence, treatment of recurrence, follow-up time in relation to initial treatment.All of the MRI scans were assessed by a single experienced radiologist who was blind to subject status and specialised in ENT and facial bone imaging.
Radiological interpretation was compared with anatomopathological results of biopsies performed in suspicious areas, thus determining statistical measures of sensitivity (Se), specificity (Sp), negative predictive value (NPV) and positive predictive value (PPV).
Results
Study population [Table 1]
We included ADK (n=24), only men, diagnosed at an advanced stage (37.5% with T4; n=9/24) and initially treated with endonasal endoscopy (75%; n=18/24). Mean follow-up was 38 months.
Postoperative MRI
The first postoperative follow-up MRI scan was performed at a mean of 65 days after surgical resection (median 50 days) with a progressive decrease in interval length over a period of years [Figure 1].
-
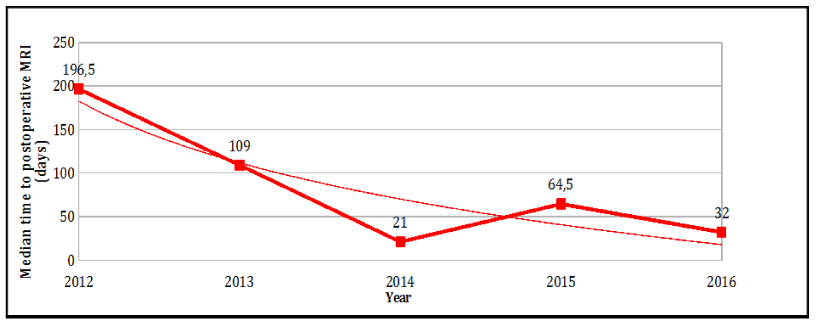
Figure 1:
Median time to postoperative MRI.
During follow-up of our 24 patients, 32 initial postoperative imaging scans were performed following initial surgery or secondary surgery for recurrence. Of the 32 imaging scans, 41% (n=13/32) showed suspicious images evoking recurrence. 69% (n=9) of the suspicious findings in these 13 MRI scans had suspicion of recurrence confirmed histopathologically and 5 cases of recurrence were observed following an initial postoperative MRI scan which appeared unremarkable (Se 64%; Sp 78%; PPV 69%; NPV 74%).
4 patients had multiple episodes of recurrence during follow-up [Figure 2].
-
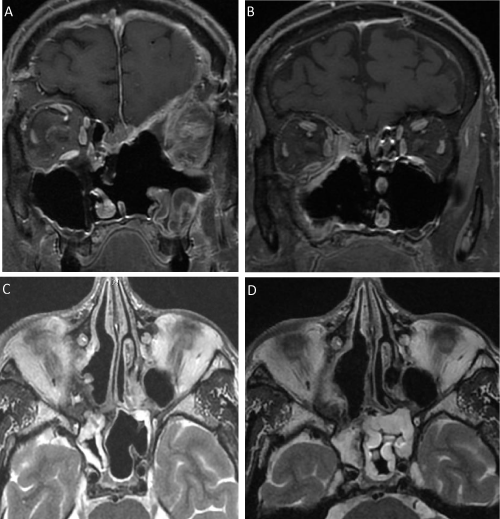
Figure 2:
Recurrence (A. and C.) and false recurrence (B. and D.) imaging. A. Coronary section T1 sequence with Gadolinium injection, post-operative J48 (left ethmoido-orbital tumoral residue and ethmoidal superior median and paramedian residue). B. Coronary section T1 sequence with Gadolinium injection, post-operative J23 (aspect of right-handed Onodi cell remainder extended along the postero-internal wall of the orbit to the anterior part of the right sphenoid. C. Axial section T2 sequence without injection, post-operative J50 (infero-medial border residue of the right orbit extend to the right Onodi cell). D. Axial section T2 sequence without injection, post-operative J23 (aspect of right-handed Onodi cell remainder extended along the post-internal wall of the orbit to the anterior part of the right sphenoid).
In the 8 patients with recurrence, 17 immediate postoperative follow-up MRI scans were indicative of recurrence. The main sites of recurrence detected are shown in Figure 3.
-
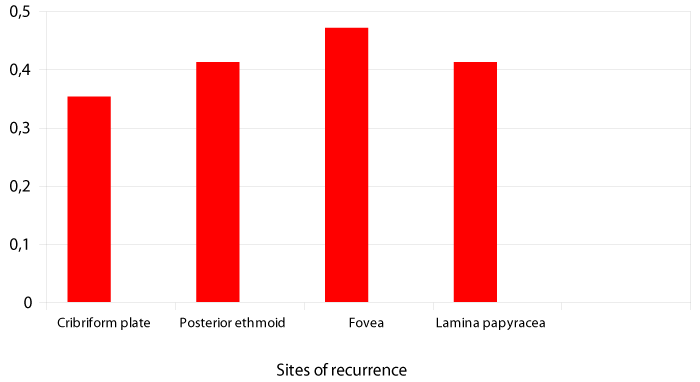
Figure 3:
Sites of recurrence.
Discussion
Current treatment of ADK is based on surgical resection followed by adjuvant radiotherapy. Rates of local recurrence remain high in the literature [5,7,8,11,14].
Within this context patient follow-up proves to be a key element in the treatment of these rare tumors.
Patient monitoring consists primarily of MRI scans and endoscopic evaluation although the exact methods involved have not been clearly defined [15].
Our retrospective monocentric study evaluates the performance of initial postoperative imaging in early detection of recurrence or of the progressive course of these tumors after surgery and the principal sites prone to risk.
Rates of recurrence in our population correspond to those found in the literature [Table 2].
-

Table 2:
Recurrence in literature.
De Gabory et al. [11] in their 2010 study detected a mean recurrence rate involving ADK of 30% in the literature, in accordance with that of our study.
The low recidivism rates found in recent studies by Vergez et al. [14] (17.6%) and Nicolai et al. [8] (21.3%) compared with the common literature (30% on average) and in our study (33.3%) can be explained in part by the presence of smaller populations in their populations of advanced tumor stages (26.1% % Of tumors classified T4 for Vergez and al., 32% of T4 tumors for Nicolai and al., 37.5% in our population). The tumor recurrence rate thus appears to be directly correlated with the initial stage of the tumor, the authors with the lowest recurrence rate being those with the least number of advanced tumors.
While it is now established that ADK of the ethmoid sinus originates in the olfactory cleft, very few studies have sought to assess the main sites prone to risk of recurrence after surgery [3-4]. Postoperative imaging findings indicate that the olfactory cleft is rarely involved in recurrence, most likely due to its access during endoscopic surgery, whereas in our study the fovea ethmoidalis (47%; n=8/17), the posterior ethmoid (41%; n=7/17) and the lamina papyracea, essentially in its inferior part (41%; n=7/17) are sites of predilection for recurrence of ADK [Figure 3].
Camp et al. [7] also found favored sites of recurrence in the posterior ethmoid (45%), the fovea ethmoidalis (35%), but equally in the posterior part of the nasal septum (27%), not found in our study population.
The main objectives underlying the acquisition of postoperative follow-up MRI imaging of ADK are twofold: i) to provide post-procedural reference scans as part of patient follow-up; ii) to carry out early detection of recurrence or progressive disease course.
By comparing suspicious postoperative imaging with anatomopathological results of biopsies taken from the corresponding suspicious areas, we obtained the statistical performance of MRI scans in the detection of recurrence or progressive disease course in these carcinomas.
Statistical performance measures, namely sensitivity (Se 64%) and negative predictive value (NPV 74%), would appear to be inadequate in establishing optimal detection of recurrence or progressive disease course at the first postoperative examination.
Due to such poor performance, endoscopic evaluation in all suspicious areas is therefore indispensable under at the minimum local anesthetic, or even under general anesthetic if the area is painful, difficult to visualize or requires the ablation of tenacious scabs.
Bely et al. [15], in a study which is now slightly dated, highlighted the necessity for initiation of ethmoid neoplasia monitoring by systematic acquisition of reference scans 3 months after surgical resection. Although the exact methods of follow-up care in these tumors are not standardized, this is the universally accepted time frame for the acquisition of initial follow-up imaging.
We have highlighted a progression towards the systematic reduction in length of time to first acquisition of postoperative follow-up MRI scans [Figure 1]. Our stance at this point is to perform MRI scans as early as possible, ideally in the week after surgical resection, so as to ensure maximally early surgical revision in the event of suspicious lesions. This stance is justified by the need to optimize local tumor control, which is a key factor in patient prognosis.
Early postoperative MRI imaging is now scheduled in our department prior to surgical procedure.
This standpoint in relation to monitoring has yet to be assessed in a prospective multicenter study, involving larger populations and with greater follow-up, in order to evaluate long-term survival in terms of benefit. However, the low sample size of the various groups hampers data exploitation of this kind.
In the interests of improving local disease control, new horizons also need to be investigated.
Consequently, since March 2015 we have been attempting to assess the use of local chemotherapy. 37.5% (n=9) of the 24 patients included have already benefited from the treatment. To date, purely subjective outcomes have so far appeared encouraging but require greater long-term evaluation in a wider population, incorporating the same difficulties as those highlighted above.
Chemotherapy, using 5-Fluorouracil, is applied topically on a nasal pack at the end of a complete surgical resection procedure as opposed to a suspicion of residual tumor. It remains in place for one week and is then renewed 2 or 3 times so as to eliminate any possible microscopic residue.
Knegt et al. [16] suggested this approach in a prospective study in 2001 in which he outlined significant improvement in the overall 5 year survival rate in the group treated with topical chemotherapy after surgery (87% survival in the surgery plus topical chemotherapy group versus 39 to 59% in the surgery alone group).
In the same way, the progressive introduction of imaging-guided surgery since the development of the late 1980s must theoretically contribute to the improvement of local control of cancer pathology by always allowing theoretically to facilitate complete removal of the tumor process. His interest appears real in advanced tumor stages where noble structures such as the orbit or the skull base are affected [17].
In our population, we used this aid for 25% of initial surgery cases and 22% of recurrence tumor cases. It is hard to say whether more frequent use of this tool had reduced our recurrence rates. In fact, Dalgorf et al., in a recent meta-analysis [18], were unable to demonstrate a statistically significant benefit for the use of imaging-guided surgery on the need for surgical revision (RR = 0.72) while this use is associated with a reduction in the rate of complications. It was also the same in the study of Ramakrishnan et al. [19]. Thus the recommendations [20] that have been published on the use of imaging-guided surgery are based on a low level of proof and the exact place of this tool remains to be defined according to the experience of the operators.
Conclusion
To our knowledge, this study is the first of its kind to attempt an objective evaluation of MRI performance in early detection of recurrence or tumor residue in carcinoma of the ethmoid sinus and to identify the predominant sites prone to risk [7].
In our department we now recommend that this investigation should be performed systematically in the week after tumor resection, and followed by immediate surgical revision in the event of the slightest doubt.
This investigation will then be repeated every 3 months during the first 3-year follow-up period, along with clinical follow-up under local or general anesthetic in the event of the slightest doubt with respect to insufficient sensitivity and specificity on MRI imaging.
This approach has yet to be approved in terms of patient survival benefit.
Finally, certain areas seem particularly prone to risk of recurrence and should as such be subject to particular consideration during initial tumor resection.
-
-
-
- PE, Powell DJ, Stansbie JM (1979) Carcinoma of the nasal cavity and paranasal sinuses: incidence and presentation of different histological types. Clin Otolaryngol Allied Sci 4: 431–456. Link: https://goo.gl/qw40Il
- Klintenberg C, Olofsson J, Hellquist H, Sokjer HS (1984) Adenocarcinoma of the ethmoid sinuses. A review of 28 cases with special reference to wood dust exposure. Cancer 54: 482–488 Link: https://goo.gl/m3gFjC
- Jankowski R, Georgel T, Vignaud JM, Hemmaoui B, Toussaint B, et al. (2007) Endoscopic surgery reveals thatwoodworkers’ adenocarcinomas originate in the olfactory cleft. Rhinology 45: 308–314. Link: https://goo.gl/tvHJEN
- Georgel T, Jankowski R, Henrot P, Baumann C, Kacha S, et al. (209) CT assessment of woodworkers' nasal adenocarcinomas confirms the origin in the olfactory cleft. AJNR Am J Neuroradiol 30: 1440-4144. Link: https://goo.gl/6q0IFB
- Choussy O, Ferron C, Védrine PO, Toussaint B, Liétin B, et al. (2008) Adenocarcinoma of Ethmoid: a GETTEC retrospective multicenter study of 418 cases; Laryngoscope. 118: 437-443. Link: https://goo.gl/W3POKb
- Lund VJ, Stammberger H, Nicolai P, Castelnuovo P, Beal T, et al. (2010) European position paper on Endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhino Suppl 1: 1-143. Link: https://goo.gl/Mz5MRp
- Camp S, Van Gerven L, Poorten VV, Nuyts S, Hermans R, et al. (2016) Long-term follow-up of 123 patients with adenocarcinoma of the sinonasal tract treated with endoscopic resection and postoperative radiation therapy. Head Neck 38: 294-300. Link: https://goo.gl/D123Ee
- Nicolai P, Schreiber A, Bolzoni Villaret A, Lombardi D, Morassi L, et al. (2016) Intestinal type adenocarcinoma of the ethmoid: Outcomes of a treatment regimen based on endoscopic surgery with or without radiotherapy. Head Neck 38: E996-E1003. Link: https://goo.gl/Ec2YNI
- Meccariello G, Deganello A, Choussy O, Gallo O, Vitali D, et al. (2016) Endoscopic nasal versus open approach for the management of sinonasal adenocarcinoma.A pooled-analysis of 1826 patients; Head Neck 38: E2267-2274. Link: https://goo.gl/uopMMY
- Moya-Plana A, Bresson D, Temam S3, Kolb F4, Janot F, et al. (2016) Development of minimally invasive surgery for sinonasal malignancy. Eur Ann Otorhinolaryngol Head Neck Dis 133: 405-411. Link: https://goo.gl/zRqHht
- de Gabory L, Maunoury A, Maurice-Tison S, Merza Abdulkhaleq H, Darrouzet V, et al. (2010) Long-term singlecenter results of management of ethmoid adenocarcinoma: 95 patients over 28 years. Ann Surg Oncol 17: 1127-1134. Link: https://goo.gl/Oq9Tkc
- Lund VJ, Stammberger H, Fokkens WJ, Beale T, Bernal-Sprekelsen M, et al. (2014) European position paper on the Anatomical terminology of the internal nose and paranasal sinuses. Rhino Suppl 1-34. Link: https://goo.gl/Bov8Q9
- Turner JH, Reh DD (2012) Incidence and survival in patients with sinonasal cancer. A historical analysis of population-based data. Head Neck34: 877-885. Link: https://goo.gl/7pbdqN
- Sebastien Vergez, Marie Devars du Mayne, Andre Coste, Patrice Gallet, Roger Jankowski, et al. (2014) Multicenter study to assess endoscopic resection of 159 sinonasal adenocarcinomas. Ann Surg Oncol 21: 1384-1390. Link: https://goo.gl/iYlNwD
- Bely N1, Zanoun M, Laccourreye O, Halimi P (1997) Radiological surveillance of operated ethmoidal cancers. Practical points. Neurochirurgie 43: 76-84. Link: https://goo.gl/wLJ82o
- Knegt PP, Ah-See KW, vd Velden LA, Kerrebijn J (2001) Adenocarcinoma of the ethmoidal sinus complex: surgical debulking and topicalfluorouracil may be the optimal treatment. Arch Otolaryngol Head Neck Surg 127: 141-146. Link: https://goo.gl/Wwzxj5
- Al-Qudah M (2015) Image-Guided Sinus Surgery in Sinonasal Pathologies With Skull Base/Orbital Erosion. J Craniofac Surg 26: 1606-1608. Link: https://goo.gl/7LcOE3
- Dalgorf DM, Sacks R, Wormald PJ, Naidoo Y, Panizza B, et al. (2013) Image-guided surgery influences perioperative morbidity from endoscopic sinus surgery: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 149: 17-29. Link: https://goo.gl/nTrHn4
- Ramakrishnan VR, Orlandi RR, Citardi MJ, Smith TL, Fried MP, et al. (2013) The use of image-guided surgery in endoscopic sinus surgery : an evidence-based review with recommandations. Int Forum Allergy Rhinol 3: 236-241. Link: https://goo.gl/RrdHiz
- American Academy of Otolaryngology – Head and Neck Surgery. Intra-operative use of computer aided surgery. Link: https://goo.gl/npOYvf









Table 1:
Characteristics population.
T4a
T4b
n=6
n=3
endoscopic
external
combined
Image-Guided Surgery
n=18
n=4
n=2
25% (n=6/24)
Multiple episodes of recurrence
17% (n=4/24)
Image-Guided Surgery
22% (n= 6/27)