Authors:
Diaa El Din El Hennawi, Mohamed Rifaat Ahmed*, Yasser T Madian, Mohammed T El-Tabbakh and Ibrahim Hassan Ibrahim
Department of Otorhinolaryngology, Faculty of medicine Suez Canal University, Ismailia – Egypt
Received: 13 May, 2017; Accepted: 22 July, 2017; Published: 26 July, 2017
Mohamed Rifaat Ahmed, MD, Assistant Professor, Otolaryngology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt, Tel: +201285043825; E-mail:
Din El Hennawi DE, Ahmed MR, Madian YT, El-Tabbakh MT, Ibrahim IH (2017) Evaluation the efficacy of the utilization the Imipramine for patients with allergic rhinitis. Arch Otolaryngol Rhinol 3(3): 079-082. DOI: 10.17352/2455-1759.000052
© 2017 Din El Hennawi DE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Allergic rhinitis; Imipramine; Desloratadine; Randomized controlled trial
Background:allergic rhinitis is an inflammatory process of the upper respiratory tract with a continuous symptom that last for hour or more with symptom affect patient’s work and quality of life.
Methodology: a randomized controlled trial in which we enrolled 284 patients with allergic rhinitis for periods longer than 4 days/week and for more than 4 consecutive weeks divided into two groups. Group A (n=142) received Imipramine 75 mg/day for 90 days .Group B (n=142), the control group, received desloratadine 5 mg / day for 90 days.
Results: The mean visual analogue score nasal symptoms intensity (sneezing, Nasal obstruction, watery rhinorrhea and itchy nose) showed a statistically significantly improvement in group A (treated with Imipramine) compared with group B (treated with desloratadine) after the same treatment period.
Conclusion: Imipramine could be used in treatment patients with allergic rhinitis as it is effective as an antihistaminic besides its main action in reliving psychosocial stresses.
Introduction
Allergic rhinitis is one of the major disorders affecting up to 9% of patients visiting otolaryngology outpatient clinics with chronic inflammatory airway as it is the sixth most common chronic disease affects the patients’ social life [1].
Symptoms of allergic rhinitis include nasal congestion, Nasal obstruction, watery rhinorrhea, postnasal drip, and sneezing, itchy nose, sleep disturbances, subsequent daytime somnolence and impairment of sleep and work performance [2,3].
The pale bluish congested nasal mucosa and watery nasal discharge considered as the main allergic rhinitis physical findings [4], allergic rhinitis defined as an allergic inflammatory process with symptoms continue more than 4 days per week and for more than 4 consecutive weeks affecting patient’s quality of life [5,6].
Psychological stresses play an important role by increasing allergic rhinitis symptoms and severity, might be due to the disturbance of the biological system and its effect on immunity and immune-related illnesses with an increased risk for atopy [7,8].
Imipramine is a tricyclic antidepressant of the dibenzazepine group used in the treatment of major depression with an immediate sedative and calming effects , in addition it hasantihistaminic properties by acting as an antihistaminic on H1 receptors [9].
The objective of the present study was to verify the efficacy of Imipramine as a treatment for patients with allergic rhinitis and its effect on improving patient's quality of life.
Materials and Methods
A randomized controlled study was carried out in Otolaryngology Department, Suez Canal University Hospital (Ismailia, Egypt) - between March 2007 and January 2012. The study protocol was approved by the local ethics committee and a written consent was obtained from all patients.
284 patients between 18 and 55 years old attending the ENT outpatient clinic with allergic rhinitis (more than 4 days/week and for more than 4 consecutive weeks) according to Mullol et al. 2005, criteria were included in the study [6].
Patients were excluded if they had significant co morbidities such as rhinitis medicamentosa, were receiving drugs known to induce nasal obstruction (e.g. beta blockers), or had previous turbinate or nasal surgery, hormonal therapy, occupational dust exposure, nasal masses, rhino sinusitis, were pregnant or lactating.
Study plan
A complete medical history and physical examination were obtained in all patients. Patients were subjected to assessment of allergic rhinitis symptoms using a scale of severity of nasal allergic rhinitis symptoms.
The patients underwent a complete ENT examination, nasal endoscopic examinations (using a 4 mm diameter, 0°, Hopkins II endoscope; Karl Storz, Tuttlingen, Germany), nasal and paranasal sinus CT scan if needed, skin prick test (to include only the positive skin test patients)
Randomization
A blocked randomization scheme using a computer generated random numbers prior to study commencement as follows: Opaque envelopes were numbered sequentially from 1 to 284. If the last digit of the random number was from 0 to 4, assignment was to group A (Imipramine), and if the last digit was from 5 to 9, assignment was to group B (desloratadine). The assignments were then placed into the opaque envelopes and the envelopes sealed. As eligible participants were entered into the trial, these envelopes were opened in sequential order to give each patient his or her random group assignment. The envelopes were opened just prior to the treatment prescription.
Patients were randomly divided into two groups. Group A (n=142) received Imipramine 75 mg/day for 90 days .Group B (n=142), the control group, received desloratadine 5 mg / day for 90 days.
Objective and outcome measurement assessment
The objective was to verify the efficacy of Imipramine as a treatment for allergic rhinitis aiming at improving patient’s symptoms severity and thereby quality of life.
All patients were asked to complete a questionnaire assessing their nasal symptoms at day zero and 90 days after treatment initiation using the same visual analogue scale (VAS) questionnaire with 0 indicates no symptoms and10 indicates severe and/or constant symptoms.
Data collection, allocation concealment and blinding
At the enrolment day (day 0), each participant underwent a brief interview with the physician to complete a questionnaire, and provided demographic and disease-related information. Demographic information including race and ethnicity were provided by selection from options included in the baseline questionnaire. The physicians then completed the documentation of symptoms and signs and again after 90 days following treatment initiation using the same visual analogue scale (VAS).
Statistical analysis
Data was analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as means ± SD while qualitative data were expressed as numbers and percentages. The Student’s t-test was used to compare the significance of difference for quantitative variables that followed a normal distribution. P value <0.05 was considered statistically significant for comparisons.
Results
In the present study we enrolled 284 patients who fulfilled the criteria of allergic rhinitis, the mean age of patients was36.2 ± 4.7 years and 98 (34.5%) were males.
The main symptoms among group A patients was sneezing in 136 (95.7%) while it was in 129 patients (90.8%) in group B followed by itchy nose in 115 patients (80.9%) and 121 patients (85.2%) respectively then nasal obstruction in 96 patients (67.6%) and 89 patients (62.6%) respectively and watery rhinorrhea in 88 patients (61.9%) and in 92 patients (64.7%) respectively, there was no statistical difference regarding these symptoms between the two groups. The main finding in nasal examination in group A patients was pale, bluish mucosa in 116 (81.6%) while it was in 109 patients (76.7%) in group B.
All patients had a positive skin prickle test which showed that 58 patients (20.4%) were positive to one allergen (monosensitized), whereas the remaining patients were polysensitized.
The mean intensity of nasal symptoms according to VAS before treatment among in group A patients was sneezing 8.13 while it was 8.72 in group B, nasal obstruction was 7.29and 7.11 respectively, watery rhinorrhea was 8.29 and in 8.73 respectively and itchy nose was 7.79 and 7.58 respectively without any statistically significance difference between the two groups (Table 1, Figure 1).
-
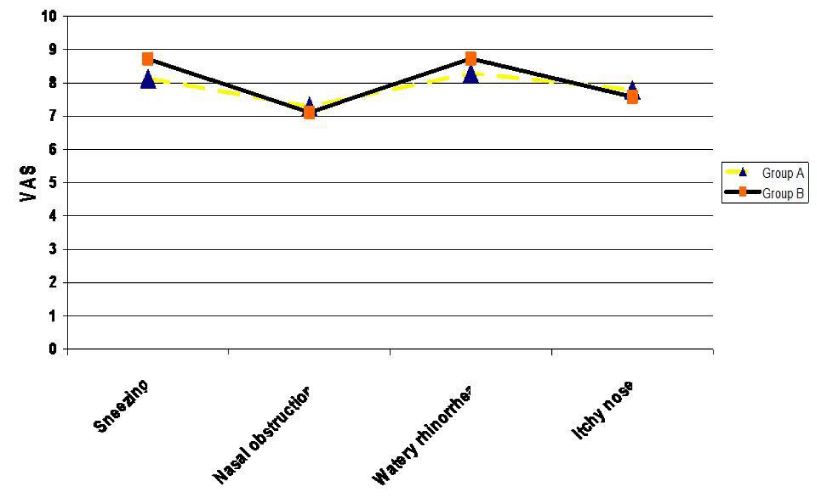
Figure 1:
Mean degree of different allergic nasal symptoms in both groups before treatment.
After three months the mean intensity of nasal symptoms according to VAS in the group A patients was sneezing (1.20) while it was 2.27 in group B, nasal obstruction was 1.08 and 2.90 respectively, watery rhinorrhea was 3.23 and in 4.05 respectively and itchy nose was 1.19 and 2.05 Respectively.
Both groups showed significant improvement at the end of the treatment period, but group A's scores were significantly better than group B's (Table 2, Figure 2-4).
-

Table 2:
Mean degree of different allergic nasal symptoms in both groups after treatment.
-
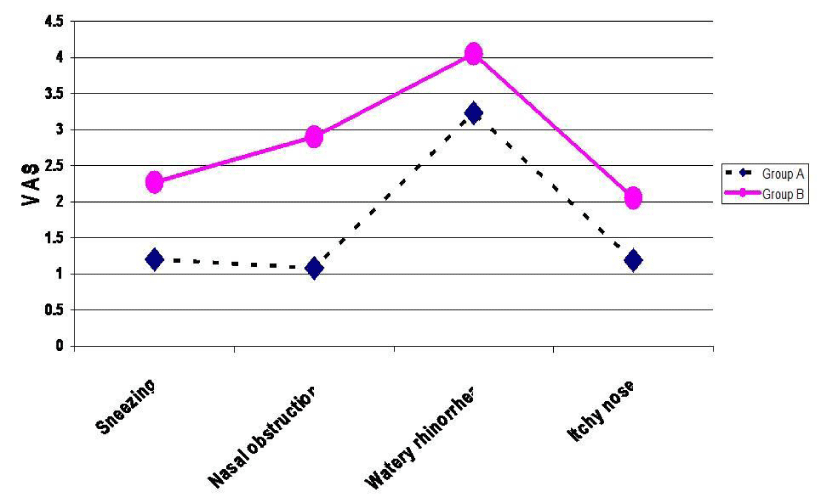
Figure 2:
Mean degree of different allergic nasal symptoms in both groups after treatment.
-
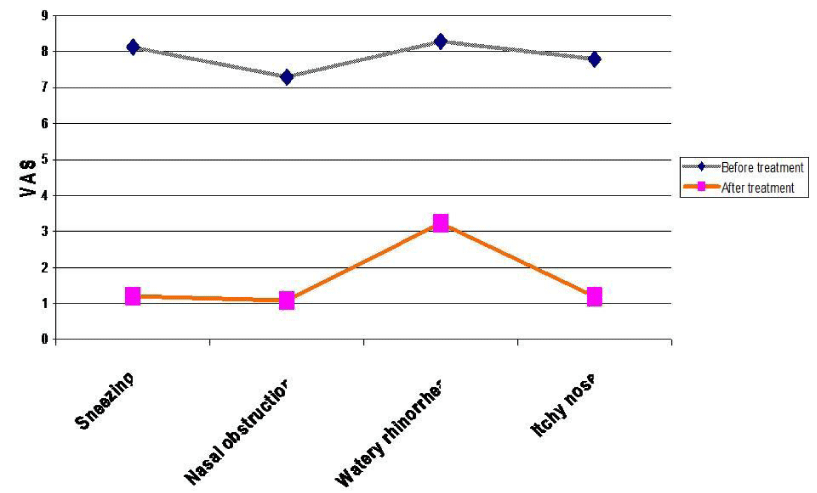
Figure 3:
Mean degree of different allergic nasal symptoms in group A before and after treatment.
-
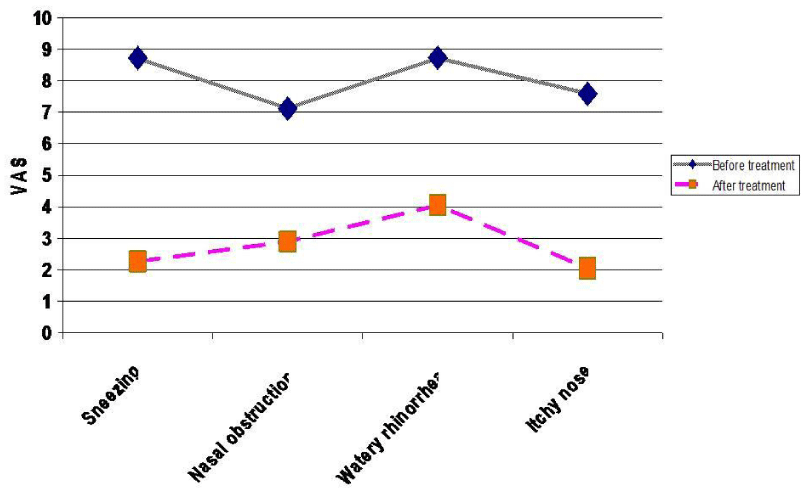
Figure 4:
Mean degree of different allergic nasal symptoms in group B before and after treatment.
There was no any adverse event in both groups during the treatment.
Discussion
Allergic rhinitis is one of the most common allergic disorders affects about 18% to 40% of general population considered as the sixth most prevalent chronic disorder in the world when ignored might cause severe complications from nasal inflammatory condition which leads to nasal obstruction and increased permeability of blood vessels sometimes accompanied with chronic rhino sinusitis, secretory otitis media, nasal polyposis, and development of bronchial asthma [10,11].
The inflammatory nature of chronic allergic rhinitis leads to nasal obstruction, difficulties in sleep awaking cycle, snoring, hyper somnolence, memory loss, diminished work performance and finally insomnia which leads to a negative impact on patient’s quality of life [12].
The mechanisms relating psychological stress with emotion to allergic rhinitis are related to hormones and neuropeptides released into the circulation when individuals experience stress and are thought to be involved in regulating both immune-mediated and neurogenic inflammatory processes [8].
The interaction of the immune, nervous and endocrine system may drive an individual to a well-recognized biological hypersensitivity and the development of allergic symptoms followed by distinct behavioral patterns characterized as affective hypersensitivity. The nervous and immune systems may interact through the action of neurotransmitters on mast cells. Both the immune and nervous systems are interacting reciprocally to affect each other [8].
Patient usually tries to overcome this situation by using the sedatives and sleeping medication which can adversely intensify the problem [13].
We found that severity of nasal symptoms (sneezing, nasal obstruction, watery rhinorrhea and itchy nose) according to VAS showed marked statistically significantly improvement in group A receiving Imipramine after the treatment period.
Imipramine affects numerous neurotransmitter systems which are involved in depression, anxiety with an action similar to the muscle relaxants, marked analgesic effect, and also an affinity for the serotonin transporter which improves sleep and quality of life. In Addition, Imipramine is an antagonist of histamine H1 receptors which contributes to the acute sedative and calming effect that happened in most people [14].
Both groups saw significant improvement at the end of the treatment period, but group A scores were significantly better than group B.
The Second -generation of antihistamines such as desloratadine considered as a potent drug for allergic rhinitis studies with increase sleepiness and improve life qualities [15].
Bousquet et al., mentioned that Continuous daily desloratadine has been shown to be effective against moderate-to-severe allergic rhinitis symptoms and improve patient’s quality of life with reverse action for impairments caused by allergic rhinitis [16].
Limitations of the present study were it was a single-center study, long follow-up period, and incomplete reporting of adverse effects of interventions with future studies needs to psychological scales evaluations for patients with allergic rhinitis.
We did not aim to create a new treatment regimen for allergic rhinitis, but our raw data showed that the benefit of administering imipramine in some patients with allergic rhinitis.
Conclusion
Imipramine could be used in treatment patients with allergic rhinitis as it is effective as an antihistaminic besides its main action in reliving psychosocial stresses.
-
-
- Wang DY, Chan A, Smith JD (2004) Management of allergic rhinitis: a common part of practice in primary care clinics. Allergy 59: 315-319. Link: https://goo.gl/uzRTtN
- Ratner PH, Ehrlich PM, Fineman SM, Meltzer EO, Skoner DP (2002) Use of Intranasal Cromolyn Sodium for Allergic Rhinitis. Mayo Clin Proc 77: 350-354. Link: https://goo.gl/nr7GeU
- Ciebiada M, Ciebiada MG, Kmiecik T, DuBuske LM, Gorski P (2008) Quality of life in patients with persistent allergic rhinitis treated with montelukast alone or in combination with levocetirizine or desloratadine. J Investig Allergol ClinImmunol 18: 343-349. Link: https://goo.gl/AHvXcN
- Nathan R (2003) Pharmocotherapy for Allergic Rhinitis: a Critical Review of Leukotriene Receptor Antagonists Compared with other Treatments. Ann Allergy Asthma Immunol 90: 182-191. Link: https://goo.gl/39EACi
- Erdoğan BA, Sanlı A, Paksoy M, Altın G, Aydın S (2014) Quality of life in patients with persistent allergic rhinitis treated with desloratadine monotherapy or desloratadine plus montelucast combination. Journal of ear, nose, and throat.">Kulak Burun BogazIhtis Derg 24: 217-224. Link: https://goo.gl/qXNEBh
- Mullol J, Bachert C, Bousquet J (2005) Management of persistent allergic rhinitis: evidence-based treatment with levocetirizine. Ther Clin Risk Manag 1: 265-271. Link: https://goo.gl/WnJPW6
- Bavbek S, Kumbasar H, Tuğcu H, Misirligil Z (2002) Psychological status of patients with seasonal and perennial allergic rhinitis. J Investig Allergol ClinImmunol 12: 204-210. Link: https://goo.gl/g8V5SJ
- Wright RJ, Cohen RT, Cohen S (2005) The impact of stress on the development and expression of atopy. Curr Opin Allergy ClinImmunol 5: 23-29. Link: https://goo.gl/fw7Juz
- Jain AK, Thomas NS, Panchagnula R (2002) Transdermal drug delivery of imipramine hydrochloride. I. Effect of terpenes. J Control Release 79: 93-101. Link: https://goo.gl/MviiXv
- Ciebiada M, Gorska-Ciebiada M, DuBuske LM, Gorski P (2006) Montelukast with desloratadine or levocetirizine for the treatment of persistent allergic rhinitis. Ann Allergy Asthma Immunol 97: 664-671. Link: https://goo.gl/JoKLk3
- Woods L, Craig TJ (2006) The importance of rhinitis on sleep, daytime somnolence, productivity and fatigue. Curr Opin Pulm Med 12: 390-396. Link: https://goo.gl/iwffy8
- Potter PC, Paediatric Levocetirizine Study Group (2005) Efficacy and safety of levocetirizine on symptoms and health related quality of life of children with perennial allergic rhinitis: a double blind, placebo controlled randomized clinical trail. Ann Allergy Asthma Immunol 95: 175-180. Link: https://goo.gl/8j15jV
- Dzaman K, Jadczak M, Rapiejko P, Usowski J, Jurkiewicz D (2005) Life quality analysis based on a self – administered questionnaire in patient with nasal obstruction. Annales UMCS 90: 416-420. Link: https://goo.gl/aqxFLM
- Lepola U, Arató M,Zhu Y, Austin C (2002) Sertraline versus imipramine treatment of comorbid panic disorder and major depressive disorder. J Clin Psychiatry 64: 654-662. Link: https://goo.gl/JtC5RL
- Nayak AS, Schenkel E (2001) Desloratadine reduces nasal congestion in patients with intermittent allergic rhinitis. Allergy 56: 1077-1080. Link: https://goo.gl/NuL7nX
- Bousquet J, Zuberbier T, Canonica GW, Fokkens WJ, Gopalan G, et al. (2013) Randomized controlled trial of desloratadine for persistentallergic rhinitis: correlations between symptom improvement and quality of life. Allergy Asthma Proc 34: 274-282. Link: https://goo.gl/igScEy
-









Table 1:
Mean degree of different allergic nasal symptoms in both groups before treatment.