Authors:
Michael Schlewet* and Peter Catalano#
Department of Otolaryngology, Head and Neck Surgery, St Elizabeth’s Medical Center, 736 Cambridge Street, SMC-8 Brighton, MA 02135, USA
Received: 06 November, 2017; Accepted: 13 November, 2017; Published: 14 November, 2017
Department of Otolaryngology, Head and Neck Surgery, St Elizabeth’s Medical Center, 736 Cambridge Street, SMC-8 Brighton, MA 02135, USA, Tel: 617-779-6440; Fax: 617-779-8483; E-Mail:
Schlewet M, Catalano P (2017) Effects of a plant based Biodegradable Middle Meatal Dressing after Endoscopic Sinus Surgery: A Prospective Comparative Study. Arch Otolaryngol Rhinol 3(4): 121-125. DOI: 10.17352/2455-1759.000062
© 2017 Schlewet M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Biodegradable; Middle meatal dressing; Intranasal stent; Endoscopic sinus surgery; NexPak; NasoPore
Background: The benefit from biodegradable middle meatal dressings is well established, and includes improved wound healing, stabilization of the middle turbinate, reduced granulation tissue, and improved patient comfort. This study compares the outcomes of a 100% plant-based nasal dressing to a popular fully synthetic dressing in patients undergoing endoscopic sinus surgery.
Design and Methods: A prospective study compared the clinical outcomes of two groups of patients undergoing endoscopic sinus surgery (ESS); the study group had a plant-based nasal dressing placed into the middle meatus, and the control group received a fully synthetic product. Outcome metrics included the degree of middle meatal crusting, amount of retained material during follow-up, need for debridement, wound healing, incidence of epistaxis, rate of post-operative infection, and patient comfort. Data was collected at 2 and 12 weeks after surgery. All patients underwent ESS for CRS and were matched for demographics and extent of disease.
Results: 25 patients were enrolled in the study group (50 dressings); the control group included 20 patients (40 dressings). The study group showed a statistically significant reduction (p<.05) in nasal crusting, amount of retained implant, patient comfort, and the need for debridement compared to the control group. However, the rate of infection was slightly higher in the study group at 2 weeks.
Conclusion: Plant based middle meatal dressings appear superior to fully synthetic dressings with regards to wound healing, crusting, rate of fragmentation, and patient comfort. A slightly elevated incidence of infection was noted.
Introduction
Endoscopic sinus surgery (ESS) is widely accepted as the gold standard of surgical treatment for sinus disease [1]. Optimal wound healing postoperatively is critical for successful outcomes. Common practice to optimize wound healing has been the use of middle metal dressings and postoperative debridement. Biodegradable middle meatal (MM) dressings have been shown to improve ESS outcomes by providing hemostasis, reducing synechia, and stabilizing the middle turbinate [2,3]. Suboptimal healing post ESS is associated with recurrent disease, scarring causing ostial or middle meatal obstruction, and recurrent infections.
Several studies have evaluated a number of biodegradable middle meatal dressings showing good clinical outcomes [3-6]. A few examples include carboxymethylcellulose foam, collagen-based Gelfoam, hyaluronic acid based film, and polyurethane sponges. However, some of these products have been associated with increased crusting, discomfort, and the need for aggressive debridement. One such example is a fully synthetic polyurethane sponge that has excellent biocompatibility and stabilizes the middle turbinate well, however, resorption is delayed (>7 days), the product is relatively stiff, and ease of use remains an issue among surgeons [4-6]. In particular, the polyurethane sponge is fully expanded at the time of insertion and therefore blocks the surgeon’s view of the target during placement.
A natural 100% plant-based middle meatal dressing (NexPak) is now available for use during ESS. Plant based polysaccharide dressings have been shown to have favorable wound healing and hemostatic properties [7-9]. We herein compare the clinical outcomes between a middle meatal dressing made with natural plant-based polysaccharides (Modified Amylopectin + Hydroxyethylcellulose) that is biocompatible, bioresorbable, and free of animal proteins, with a fully synthetic polyurethane foam dressing (NasoPore).
Study Design
This is a prospective comparative study of 45 patients who required ESS due to medically refractory CRS with or without nasal polyps per AAO-HNS chronic rhinosinusitis diagnostic criteria guidelines [10].
45 patients who met the criteria of chronic rhinosinusitis with or without nasal polyposis (CRSw/sNP) were enrolled. Patient selection into the two different groups was by simple randomization. 25 patients were in the study group and received the plant-based dressing, and another 20 patients were in the control group and received the polyurethane dressing (Table 1). The dressing was placed intra-operatively in the middle meatus after completion of ESS and was hydrated with Bacitracin solution. Nasal saline irrigations were begun on post-op day #1 and continued BID in all patients for 30 days. No patient received oral antibiotics, antihistamine, oral or topical steroids during the study period.
As was previously shown [5,6,11], the topical targeted delivery of Bacitracin was as effective as oral antibiotics in the prevention of post-surgical sinusitis. Furthermore, the effect of topical Bacitracin on the healing process, infection, and hemostasis, if any, will be the same in both groups, and shall not be considered a confounding factor.
During their 2 and 12 weeks follow-up visits after surgery, patients underwent nasal endoscopic evaluation for the following parameters: degree of crusting, amount of retained implant, infection, allergic reaction to the dressing, and presence of adhesion/synechia. These parameters were graded as follow: Crusting -none (0), mild (1), moderate (2), severe (3). Retained implant was graded as either none (0) or present (1). A history was also taken with respect to post-op epistaxis and level of comfort.
To minimize observer bias, postoperative nasal endoscopic evaluations were performed by three independent otolaryngologists who were not directly involved with the primary surgery. Both groups underwent the same surgical procedure: septoplasty, bilateral uncinectomy, bilateral anterior ethmoidectomy, and bilateral radiofrequency ablation of inferior turbinates.
Results
The assessment of degree of crusting was described as none (0), mild (1), moderate (2), and severe (3). (Table 2, Figures 1,2).
-

Table 2:
Degree of crusting at 2-weeks (number of dressings and percentage for each group).
-
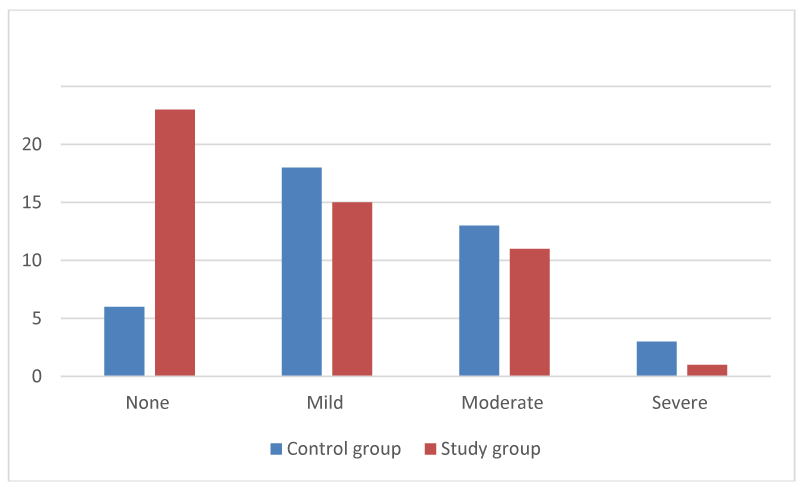
Figure 1:
Degree of crusting at 2-weeks (number of dressings matched to degree of crusting).
-
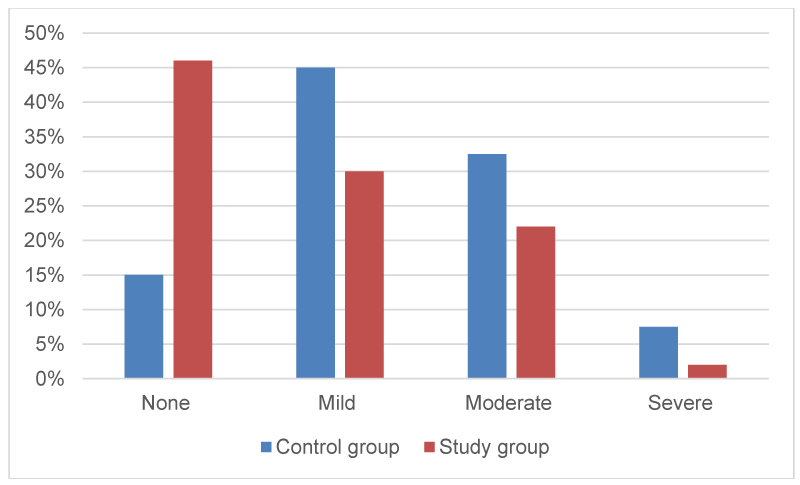
Figure 2:
Degree of crusting at 2-weeks (percentage among each group).
For the study group, the average degree of crusting (values from 0 to 3) was 0.8 (STD dev 0.86), indicating mostly None to Mild crusting at 2-weeks post ESS. For the control group, the average degree of crusting was 1.32 (std dev 0.83) indicating mostly mild to moderate crusting at 2-weeks post ESS (Figure 3).
-
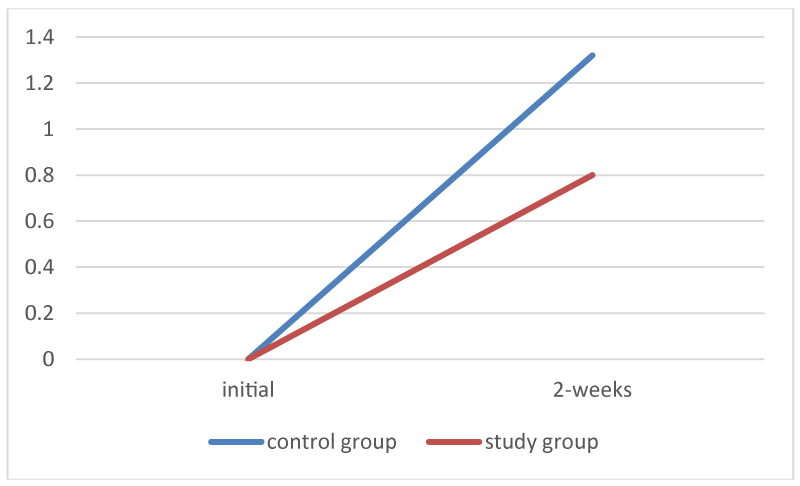
Figure 3:
Average of degree of crusting in each group.
Independent t-test confirmed that the difference in the degree of crusting between the two groups was statistically significant (p< 0.005).
The assessment of any retained dressing was described as either none (0) (no retained material found), and present (1) (retained material found) (Table 3, Figures 4,5).
-

Table 3:
Retained implant at 2-weeks (number of implants and percentage among each group).
-
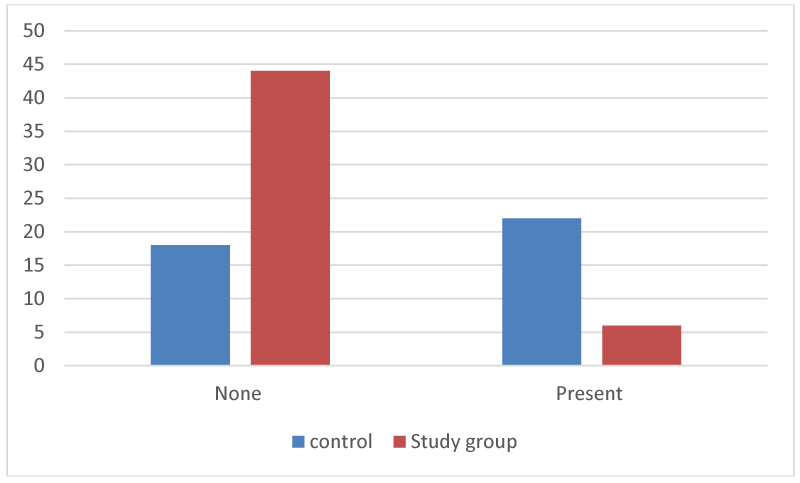
Figure 4:
Retained implant at 2-weeks (number of implants matched to retained implant).
-
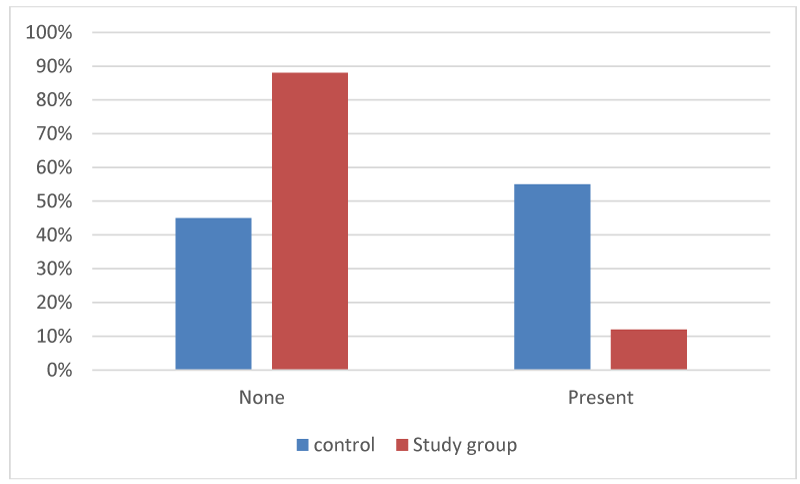
Figure 5:
Retained implant at 2-weeks (percentage among each group).
The average score for retained material at 2-weeks for the study group was 0.12 (std dev 0.33), whereas the average for the control group was 0.55 (std dev 0.5). This indicates faster resorption of the plant based dressing compared to the polyurethane implant. (Figure 6).
-
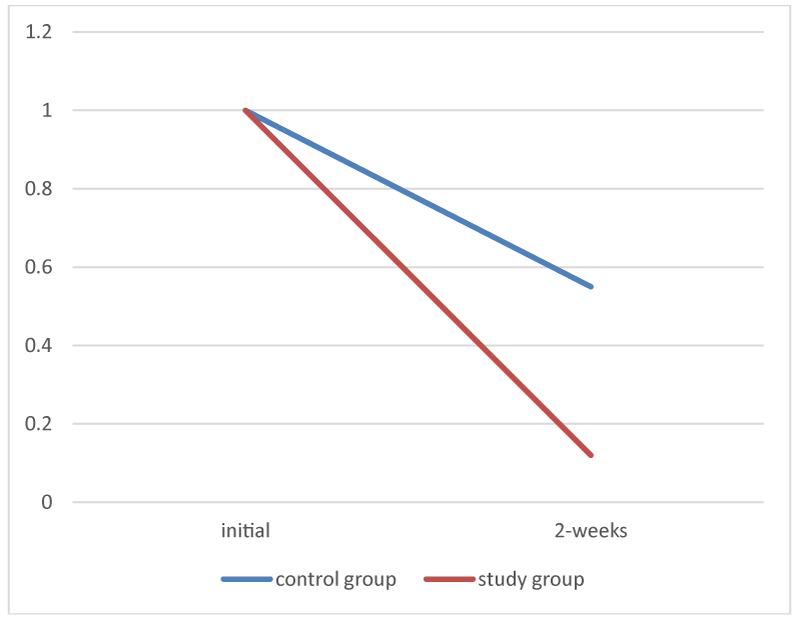
Figure 6:
Average of retained implant in each group.
An independent t-test showed that the difference in the amount of retained dressing between the two groups was highly significant (p< 0.0001).
The need for post-op endoscopic debridement between the two groups was also compared and showed that 55% of the control group required endoscopic debridement compared to 36% of the study group (Table 4).
-

Table 4:
Requirement of post-op endoscopic debridement.
Three of 20 patients (15%) in the control group with normal pre-op coagulation profile had peri-operative epistaxis requiring medical or surgical intervention, whereas 2 of 25 patients (8%) in the study group with a normal pre-op coagulation profile developed epistaxis requiring treatment (Table 5).
-

Table 5:
Post –op epistaxis incidence.
No patient in either group exhibited an allergic reaction, adhesions, or synechiae in the 12 weeks follow-up period.
We noted a higher incidence of post-op infection and subsequent need for oral antibiotics in the study group (16%) versus the control group (5%) at 2 weeks (Table 6).
-

Table 6:
Post –op infection incidence at 2 weeks.
There was no clinical difference between groups with respect to the synechiae within the middle meatus, or the rate of recurrent sinusitis at 12 weeks.
Discussion
Over the past decade, multiple biodegradable nasal dressings were widely used in stenting the middle meatus after ESS to improve wound healing and hemostasis, and to reduce adhesion. Several clinical studies compared the various products with each other, with non-resorbable nasal dressings, and with no nasal dressing at all [2]. These studies showed differences between the various types of biodegradable dressing, with some products demonstrating superiority in certain aspects, and inferiority in others. For instance, Floseal was superior for hemostasis, but caused an increase in middle meatal adhesions [12]. Hyaluronic acid dressings were shown to form inclusion bodies in the mucosal surface of the surgical wound, leading to a foreign body reaction [5]. Both products showed poor biocompatibility resulting in increased crusting, delayed wound healing, and reduced patient comfort and satisfaction after ESS [3,5,13].
On other hand, polyurethane foam nasal dressing used herein was found to have better biocompatibility, with improved wound healing and hemostasis compared to other types absorbable nasal dressings [4]. It has, therefore, become a very popular nasal dressing [4,6]. In the past 2 years, a Chitosan-based resorbable polymer dressing became available and was found to outperform the polyurethane foam nasal dressing [6]. There are additional studies showing favorable outcomes related to Chitosan’s positive influence on wound healing, hemostasis, and antimicrobial properties [14,15]. Despite these favorable properties, there are a number of patients and providers who have concerns regarding the animal-based sourcing of Chitosan.
A purely plant-based option for nasal dressing has recently become FDA approved. The plant based nasal dressing is sourced from modified purified potato starch (amylopectin + hydroxyethyl cellulose). This product is biodegradable, has been shown to minimize bleeding, helps prevent adhesions, and has been used in other surgical settings with excellent results [9].
In this study we aimed to compare this new plant based nasal dressing (NexPak) with the well-studied polyurethane dressing (Nasopore) to assess its efficacy when used in the middle meatus after ESS. The study and control groups had very similar demographic and medical profiles. In this investigation the dressings were hydrated with Bacitracin solution, as it has been done in a previous comparative study [6]. The use of topical antibiotic in a bio-absorbable dressing after ESS has been shown by Wijewickrama et al., to significantly reduce the need for systemic antibiotics postoperatively [11]. Elimination of post-operative systemic antibiotics is a cost-effective alternative, eliminates concerns of antibiotic side effects, drug-drug interaction, and patient medication compliance in the postoperative period.
The results of our study demonstrate the superiority of the plant-based dressing versus a polyurethane sponge regarding the degree of MM crusting (P< 0.005), product resorption (P< 0.0001), and a lower incidence of peri-operative epistaxis.
However, we did see an increase in incidence of post-op sinusitis in the study group versus controls (16% vs 5%). This is possibly due to the fact that plant-based polysaccharides do not have antimicrobial properties as seen with Chitosan.
A shortcoming of this study is the subjective nature of the endoscopy scores with respect to degree of crusting and retained dressing material at follow-up. We attempted to eliminate observer bias by not allowing the operating surgeon to score any patient during the post-op period. We also eliminated any pharmacologic confounders by preventing the use of immune modulating drugs during the study. The study size is consistent with other similar investigations.
Conclusion
A plant based polysaccharide-based nasal dressing (NexPak) demonstrated a statistically significant improvement over the polyurethane nasal dressing (Nasopore) in postoperative wound healing characteristics, including the degree of MM crusting, amount of retained MM dressing, and reduced peri-operative epistaxis in patients undergoing ESS for CRS. Patients receiving the plant polysaccharide-based dressing also required much less postoperative debridement, which directly relates to patient comfort. There was a higher rate of peri-operative sinusitis in the plant polysaccharide-based group compared to controls.
-
-
-
- Stammberger H, Posawetz W (1990) Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol 247: 63-76. Link: https://goo.gl/KZNDTr
- Yan M, Zheng D, Li Y, Zheng Q, Chen J, et al. (2014) Biodegradable nasal Packings for Endoscopic Sinus Surgery: A Systematic Review and Meta-Analysis. PLoS One 9:e115458. Link: https://goo.gl/2ubXp1
- Cote DW, Wright ED (2010) Triamcinolone-Impregnated Nasal Dressing Following Endoscopic Sinus Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. Laryngoscope 120: 1269–1273. Link: https://goo.gl/mXq7SH
- Catalano PJ, Payne S, Thong M (2011) Clinical Evaluation of a Fully Synthetic Middle Meatal Stent for Safety and Tolerability. Otolaryngol.–Head Neck Surg 144: 452–456. Link: https://goo.gl/w78LGw
- Catalano PJ, Roffman E (2003) Evaluation of middle meatal stenting after minimally invasive sinus techniques (MIST). Otolaryngol.–Head Neck Surg 128: 875–881. Link: https://goo.gl/VXKi6T
- Hsu K, Erickson M, Catalano P (2015) Effect of a chitosan-based biodegradable middle meatal dressing after endoscopic sinus surgery: A prospective randomized comparative study. Sinusitis 1: 3-12. Link: https://goo.gl/ALb8WC
- Antisdel JL, Matijasec JL, Ting JY, Sindwani R (2011) Microporous polysaccharide hemospheres do not increase synechiae after sinus surgery: randomized controlled study. Am J Rhinol Allergy 25: 268-271. Link: https://goo.gl/V1sjRh
- Antisdel JL, West-Denning JL, Sindwani R (2009) Effect of microporous polysaccharide hemospheres (MPH) on bleeding after endoscopic sinus surgery: randomized controlled study. Otolaryngol Head Neck Surg 141: 353-357. Link: https://goo.gl/o9CxiC
- Kilbourne M, Keledjian K, Hess JR, Scalea T, Bochicchio GV (2009) Hemostatic efficacy of modified amylopectin powder in a lethal porcine model of extremity arterial injury. Ann Emerg Med 53: 804-810. Link: https://goo.gl/zYZBVb
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K et al. (2015) Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg 152: S1-S39. Link: https://goo.gl/XoB7N5
- Wijewickrama R, Catalano P, Gupta R, Willen S, More Y, et al. (2013) Efficacy of targeted middle metal antibiotics and endoscopic sinus surgery. Am J Rhinol Allergy 27: 329–332. Link: https://goo.gl/QTxfex
- Weitzel EKm, Wormald PJ (2008) A scientific review of middle meatal packing/stents. Am J Rhinol 22: 302–307. Link: https://goo.gl/VjtiXF
- Kastl KG, Reichert M, Scheithauer MO, Sommer F, Kisser U, et al. (2014) Patient comfort following FESS and NasoPore Packing, A double blind, prospective, randomized trial. Int J Rhinol 52: 60–65. Link: https://goo.gl/zim4gQ
- Ong SY, Wu J, Moochhala SM, Tan MH, Lu J (2008) Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 29: 4323–4332. Link: https://goo.gl/r2BDHY
- Huang X, Sun Y, Nie J, Lu W, Yang L, et al. (2015) Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int J Biol Macromol 75: 322–329. Link: https://goo.gl/oWyDdY
-
-
-









Table 1:
Demographics and patients characteristics.