Authors:
Michael S Balzer#, Janina Müller-Deile#, Daniela I Schulze#, Georg Eisenbach, Bernhard MW Schmidt, Hermann Haller and Roland Schmitt*
Division of Nephrology and Hypertension, Department of Medicine, Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany
Received: 28 October, 2016; Accepted: 29 November; Published: 30 November, 2016
Roland Schmitt, MD, Hannover Medical School, Carl-Neuberg-Str. 1, 30625, Hannover, Germany, Tel: +49 511 532 6322; Fax: +49 511 600 604 35; E-mail:
Balzer MS, Müller-Deile J, Schulze DI, Eisenbach G, Schmidt BMW, et al. (2016) Potential Impact of Dialysate Magnesium on Intradialytic Hypotension. Arch Renal Dis Manag 2(1): 031-034. DOI: 10.17352/2455-5495.000014
© 2016 Balzer MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Blood Pressure, Dialysate, Hemodialysis, Intradialytic Hypotension, Magnesium
Numerous beneficial effects on cardiovascular health have been described for magnesium (Mg). Intradialytic hypotension (IDH) is a common complication in hemodialysis patients which contributes to cardiovascular mortality. It has been suggested that higher dialysate Mg (DMg) might reduce the risk of IDH. In this 24-month retrospective observational analysis we studied the incidence of IDH in 45 stable hemodialysis patients who underwent conversion from 0.5 mmol/L DMg to 1.0 mmol/L. Mean serum Mg was 1.12 ± 0.03 mmol/L pre-conversion and changed to 1.35 ± 0.04 mmol/L post-conversion. In parallel, incidence of IDH showed a significant decrease from 1.59 ± 0.34 to 1.08 ± 0.27 % comparing 12 months before and 12 months after DMg conversion. The incidence of muscle cramps and serum calcium showed a trend for reduction while other parameters were unchanged. In conclusion, switching patients from 0.5 mmol/L DMg to 1.0 mmol/L was associated with a significant improvement of IDH incidence without significant changes in other parameters that were analyzed in this study. Further studies are warranted to test the association between DMg and IDH in a prospective randomized fashion.
Introduction
Worldwide, hemodialysis is the most commonly used treatment modality for patients with end-stage renal disease. Despite technological advances in the delivery of hemodialysis, the mortality rate of patients on long-term hemodialysis remains alarmingly high, with cardiovascular disease being the leading cause of death [1]. One of the most common complications of hemodialysis and an important contributor to cardiovascular mortality is intradialytic hypotension (IDH). While there is no generally accepted definition of IDH, Kidney Disease Outcomes Quality Initiative (K/DOQI) and European Best Practice guidelines define IDH as a decrease in systolic blood pressure of more than 20 mmHg in association with symptoms and/or the need for nursing interventions [2,3]. IDH may occur in up to 25% of treatments and it is assumed that the incidence will continue to increase as a growing number of elderly patients will be developing end-stage renal disease [4,5]. By causing repetitive tissue hypoperfusion, IDH has detrimental effects on several organ systems: IDH leads to a critical shortage of blood supply to the gut and subsequently to increased intestinal permeability, endotoxemia and microinflammation [6]; furthermore, IDH causes cerebral hypoperfusion and cognitive impairment [7]; due to recurrent myocardial ischemia IDH aggravates preexisting cardiac disease and promotes heart fibrosis and arrhythmia [6]. The most important trigger for IDH is a rapid decrease in effective blood volume caused by high ultrafiltration rates. However, slowing ultrafiltration as recommended by the European Best Practice guidelines is often difficult to achieve [2]. Cooler dialysate temperature is another strategy against IDH which is currently tested in clinical trials [7]. An additional option to improve intradialytic blood pressure stability is to increase sodium or calcium concentration in the dialysate [3]. A higher sodium level, however, may expand total body water and thereby aggravate ultrafiltration requirements. Higher calcium may lead to calcium loading which enhances the risk of vascular and extravascular calcification. A safer option to counteract IDH could be a higher dialysate magnesium concentration (DMg) as suggested by experimental and clinical data [8].
Mg, which is the fourth most abundant cation in the human body, has direct effects on many physiological variables, e.g. blood pressure, myocardial contractility, bone mineralization and parathyroid function [9]. Mg stabilizes enzymes in ATP generating reactions; it is important for cell adhesion and membrane transport and modulates insulin signaling [9]. About 99% of total Mg is located in bone, muscles and non-muscular soft tissue. In the general population, several studies have indicated an association between low serum Mg and ischemic heart disease, diabetes mellitus, metabolic syndrome, coronary artery disease and atherosclerosis [10,11]. Mg homeostasis is determined by dietary Mg intake, intestinal absorption and renal excretion. In hemodialysis patients this normal balance is lost and Mg homeostasis becomes majorly dependent on Mg removal during hemodialysis. As current hemodialysis guidelines do not recommend specific dialysate Mg concentrations, common standard compositions vary from 0.25 to 1.00 mmol/L Mg [12]. Correlative studies in hemodialysis cohorts suggest that higher dialysate Mg concentrations might be advantageous for cardiovascular disease and for preventing IDH [13,14]. The goal of our retrospective study was to examine the effects of increased DMg on the occurrence of IDH in an outpatient hemodialysis center in which standard treatment had been changed from 0.5 mmol/L to 1.0 mmol/L DMg.
Materials and Methods
Patient characteristics
The study was performed as a retrospective, longitudinal single-center study at Kuratorium für Dialyse und Nierentransplantation, a non-profit dialysis provider associated with Hannover Medical School. For evaluating the impact of doubling DMg from 0.5 to 1.0 mmol/L, a period of 12 months before and 12 months after dialysate conversion was analyzed. 45 patients met the inclusion criteria (age above 18 years, chronic hemodialysis for at least 3 months before study period, completion of 24 months study period, hemodialysis using the central dialysate supply system, no episodes longer than 3 weeks outside the center due to hospitalization or vacation). Patient baseline characteristics are shown in Table 1. All patients underwent three standard weekly hemodialysis treatments of 4-5 hours. According to the local standard maximum ultrafiltration rates were ≤ 13ml/kg/hour in all patients throughout the study period. Blood and dialysate flow was standardized to 300 mL/min (Qb) and 500 mL/min (Qd), respectively. Hemodialysis was performed with high-flux polysulfone filters using individualized bicarbonate levels ranging from 30-36 mmol/L. Dialysate calcium was standardized to 1.25 mmol/L. The study had been approved by the institutional ethics board of Hannover Medical School and by Kuratorium für Dialyse und Nierentransplantation.
Data evaluation
The frequency of IDH and muscle cramps was analyzed by evaluating routine patient records. IDH was defined as a drop in systolic blood pressure of more than 20 mmHg and concurrent requirement of volume expansion by nurse intervention. Muscle cramps were defined as localized, painful contractions requiring nurse intervention (generally injection of hypertonic saline). Archived treatment and laboratory data were analyzed for intradialytic weight loss by ultrafiltration, changes in calcium (Ca), phosphate (PO4) and intact parathyroid hormone (iPTH).
Statistical analysis
For comparison of paired dichotomous data, McNemar test was applied and for comparison of paired continuous variables statistical analyses comprised two-tailed paired t-tests. Analyses were performed using a web-based McNemar test and IBM SPSS Statistics 22. Results are given as means with standard error of the mean (SEM), unless otherwise stated. Changes were considered statistically significant for p < 0.05 (two-sided).
Results
Patient characteristics and impact of DMg conversion on serum Mg
Baseline characteristics of the 45 study patients are summarized in Table 1. Their mean age was 57.9 ± 17.6 years, and a total of 31 patients were male. The median duration of dialysis was 10.5 ± 8.0 years. Mean serum Mg at baseline was 1.12 ± 0.03 mmol/L, which was at the upper limit of normal (0.70-1.10 mmol/L). Conversion from standard (0.5 mmol/L) to high (1.0 mmol/L) DMg caused a significant increase in mean pre-dialysis serum Mg levels to 1.35 ± 0.04 mmol/L (p < 0.0001). Dialysate composition differed only by Mg content while all other components were not altered. The number of patients that took oral magnesium supplements were lower under high DMg compared to lower DMg (Table 2). Mg containing phosphate binders were not used during the time of the study (p < 0.0001). (Figure 1).
-

Table 2:
Clinical and routine laboratory parameters prior to and after change of Mg dialysate conversion.
-
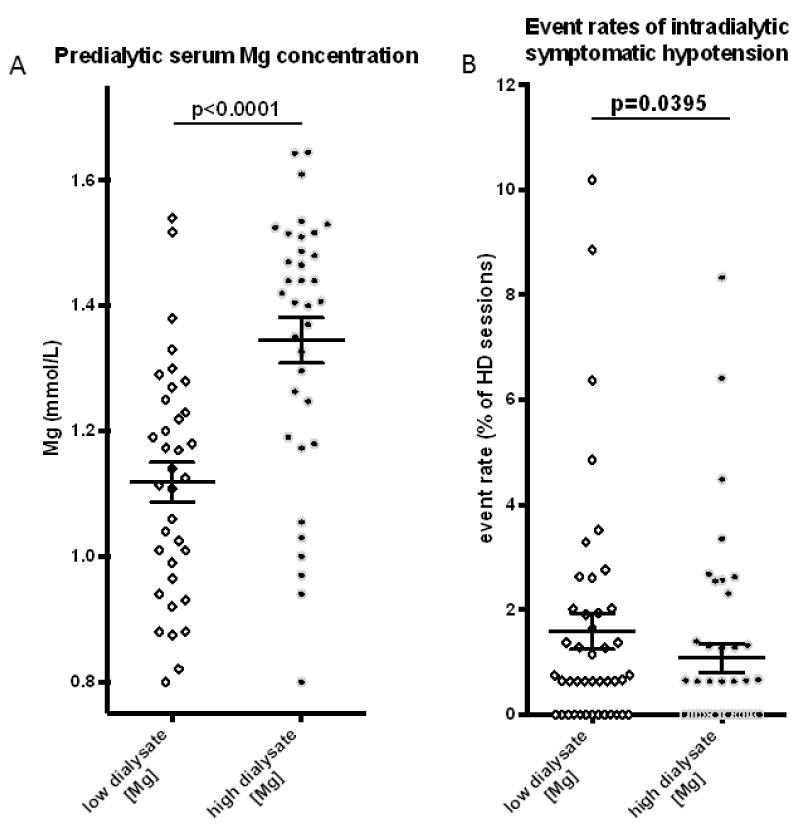
Figure 1:
Scatter plots of selected parameters from table 2 to illustrate the impact of dialysate conversion from dialysate with a Mg concentration of 0.5 mmol/L to 1.0 mmol/L on (A) predialytic serum Mg and (B) intradialytic hypotension (IDH). Apart from dialysate Mg, all other components of the dialysate remained unchanged.
Impact of DMg conversion on IDH and muscle cramps
The occurrence of IDH during the defined 24-month study period was analyzed in a total of 13480 hemodialysis sessions (150.4 ± 2.1 per patient before vs. 151.4 ± 1.8 per patient after DMg conversion, p = 0.6679). IDH as defined by a drop in systolic blood pressure of more than 20 mmHg and concurrent requirement of volume expansion occurred at least once in 75% of patients over the course of the total study period. Frequency of IDH decreased from 1.59 ± 0.34% to 1.08 ± 0.27% (p = 0.0395) after increasing dialysate Mg. In parallel, the incidence of muscle cramps with requirement for nurse intervention showed a strong trend for reduction from 9.77 ± 2.50% to 6.82 ± 2.14% but this difference did not reach statistical significance (p = 0.0942). The mean weight loss during ultrafiltration was 2.35 ± 0.78 kg per dialysis treatment vs. 2.49 ± 0.78 kg which was not significantly different between the study periods (p = 0.4363).
Impact of DMg conversion on bone metabolism parameters
With conversion to higher DMg there was a small but almost significant decrease in serum calcium from 2.22 ± 0.02 mmol/l to 2.18 ± 0.02 mmol/l (p = 0.0638), no change in serum phosphate PO4 (1.69 ± 0.05 mmol/L vs. 1.67 ± 0.03; p = 0.7399) and no change in iPTH (199.0 ± 23.5 pg/ml vs. 168.6 ± 20.1 pg/ml; p = 0.1183; Table 2).
Discussion
Given the association of IDH and cardiovascular disease related mortality in hemodialysis patients [5], targeting IDH by modulating DMg is of great potential importance. In this study, we found that increasing DMg from 0.5 to 1.0 mmol/L was associated with a reduction in IDH comparing 12 months before to 12 months after the conversion. These findings argue for a therapeutic benefit and support the concept that the blood pressure stabilizing role of Mg is also valid in hemodialysis [8].
It is important to note however, that we observed a relatively low IDH prevalence in our patients when compared to other published studies [4,5]. Apart from our stringent definition of IDH there are several reasons that might account for the lower prevalence: Despite their long average dialysis vintage (10.5 years) the comorbidity burden in our patients was moderate (e.g. diabetes mellitus below 10%) and average age was below 60 years. Additionally, ultrafiltration rates, the main risk factor for IDH, were restricted to a maximum of 13ml/kg/hour as per our center’s policy. It will be important to test whether the present findings are transferable to patient populations with higher comorbidity, higher ultrafiltration rates and higher incidences of IDH.
A large cross-sectional study investigated the correlation between serum Mg and cardiovascular mortality in a cohort of more than 140,000 hemodialysis patients [15]. The majority of patients used DMg of 0.5 mmol/L and their mean serum Mg concentration was 1.07 mmol/L, which is similar to the pre-conversion Mg in our patients (1.12 mmol/L). The study found that cardiovascular mortality significantly worsened with serum Mg levels being below 1.15 mmol/L [15]. The same threshold of 1.15 mmol/L was independently found in a study of hemodialysis patients treated with a 1.0 mmol/L DMg [16]. It was concluded that increasing serum Mg >1.15 mmol/L might have beneficial effects on cardiovascular disease related mortality. From the published data it is not clear whether IDH is involved in this mortality reduction; our data, however, suggest that this might be the case. The main limitations of our study are the single-center retrospective character and the relatively small number of patients. Mean dialysis vintage in our cohort was long and relatively few patients had diabetes mellitus, which may limit the comparability to other hemodialysis populations. Moreover, there might have been confounders such as changes in residual renal function that were not evaluated. However, despite these shortcomings our study has several strengths: In contrast to previously published cross-sectional reports our study allowed for a longitudinal patient follow-up before and after DMg conversion. This is not possible in cross-sectional cohort data in which individual serum Mg levels are influenced by a plethora of covariates (e.g. differences in nutritional status) which might have independent effects on the investigated endpoints. The relative increase of serum Mg in our patients was a direct result of the timed dialysate conversion. Independent covariates are therefore less likely to be causally involved.
So far, there are only two published studies that have analyzed the impact of increased dialysate Mg on intradialytic blood pressure stability in an interventional manner. In the first study, dialysate calcium and Mg concentrations were changed sequentially in 4-week intervals in a group of 14 hemodialysis patients [17]. The study revealed superiority of a dialysate containing 1.25 mmol/L calcium and 0.75 mmol/L Mg over a combination of 1.25 mmol/L calcium with 0.25 mmol/L or 0.5 mmol/L Mg [17]. The second study, which has only been presented in abstract form, found no benefit for intradialytic blood pressure stability after an increase in dialysate Mg from 0.5 mmol/L to 1.0 mmol/L [18]. Results of the latter study seem to differ from our own findings, though follow-up was only 2 weeks as compared to 12 months after DMg conversion in our study. Mg has well-known long-term effects in hemodialysis patients, e.g. reduced intima media thickness and lower arterial calcification burden [19,20]. It is possible that associated changes in vasomotor activity and hemodynamic stability are missed in a short observation period.
Filiopoulos and colleagues have recently reported on changes of parameters of bone metabolism during a 4 month observation in 29 hemodialysis patients after DMg conversion from 1mEq/L. to 1.5 mEq/L [21]. They found that the resulting hypermagnesemia was paralleled by a significant decrease in serum calcium with no change in iPTH or serum phosphate. In line with their findings our patients showed no changes in these parameters except a nearly statistically significant drop in calcium. However, the magnitude of this change was too small to draw any conclusions about its clinical significance.
Mechanistically, hypocalcemia could be well explained by the reciprocal interplay between Mg and calcium. For example, it has been shown that Mg supplementation reduces apparent calcium absorption but promotes bone formation and prevents bone resorption [22]. Moreover, Mg is a potent inhibitor of the calcification process [23]. Thus, the drop in serum calcium seen after increasing DMg from 0.5 to 1.0 mmol/L might have a long-term impact on bone structure. However, follow-up of the patients was not designed to evaluate for this.
In conclusion, increasing DMg led to a reduction in IDH incidence without overt side effects. Higher DMg could thus serve as an effective strategy to reduce IDH and associated cardiovascular events. However, it needs to be tested whether our retrospective single center experience can be transferred to other patient populations. Ideally, a randomized controlled trial should be performed that allows not only monitoring of DMg dependent changes in IDH but also the secondary impact on cardiovascular outcome.
- U.S. Renal Data System: Usrds 2014 annual data report: Atlas of end-stage renal disease in the United States, national institutes of health, national institute of diabetes and digestive and kidney diseases, Bethesda, MD, 2014.
- Kooman J, Basci A, Pizzarelli F, Canaud B, Haage P, et al. (2007) Ebpg guideline on haemodynamic instability. Nephrol Dial Transplant 22 ii22-44 .
- KDOQI (2005) Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients, 2015 ,
- Agarwal R (2012) How can we prevent intradialytic hypotension? Current opinion in nephrology and hypertension 21: 593-599 .
- Stefansson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, et al. (2014) Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9: 2124-2132 .
- McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, et al. (2011) Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133-141 .
- Eldehni MT, Odudu A, McIntyre CW (2014) Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957-965 .
- Sherman RA (2001) Modifying the dialysis prescription to reduce intradialytic hypotension. Am J Kidney Dis 38: S18-25 .
- Tzanakis IP, Oreopoulos DG (2009) Beneficial effects of magnesium in chronic renal failure: A foe no longer. International urology and nephrology 41: 363-371 .
- He K, Liu K, Daviglus ML, Morris SJ, Loria CM, et al. (2006) Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 113: 1675-1682 .
- Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, et al. (1999) Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The atherosclerosis risk in communities study. Archives of internal medicine 159: 2151-2159 .
- AL MdF, Rodriguez M (2013) Magnesium - its role in ckd. (Nefrologia: publicacion oficial de la Sociedad Espanola) Nefrologia 33: 389-399 .
- McIntyre CW (2008) Calcium balance during hemodialysis. Semin Dial 21: 38-42 .
- Pakfetrat M, Malekmakan L, Roozbeh J, Haghpanah S (2008) Magnesium and its relationship to c-reactive protein among hemodialysis patients. Magnesium research: official organ of the International Society for the Development of Research on Magnesium 21: 167-170 .
- Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, et al. (2014) Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 85: 174-181 .
- Joao Matias P, Azevedo A, Laranjinha I, Navarro D, Mendes M, et al. (2014) Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif 38: 244-252 .
- Kyriazis J, Kalogeropoulou K, Bilirakis L, Smirnioudis N, Pikounis V, et al. (2004) Dialysate magnesium level and blood pressure. Kidney Int 66: 1221-1231 .
- Jefferies HJ, McIntyre CW (2010) Use of high magnesium dialysate does not abrogate intradialytic haemodynamic instability or haemodialysis-induced myocardial stunning. J Am Soc Nephrol 21: 223ATH-PO490.
- Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, et al. (2014) Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PloS one 9 :e116273 .
- Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, et al. (2008) Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. International urology and nephrology 40: 1075-1082 .
- Filiopoulos V, Hadjiyannakos D, Vlassopoulos D (2016) Optimal Plasma and Dialysate Magnesium Concentrations in Hemodialysis Patients: The Unsettled Issues. Am J Kidney Dis 67: 341 .
- Toba Y, Kajita Y, Masuyama R, Takada Y, Suzuki K, et al. (2000) Dietary magnesium supplementation affects bone metabolism and dynamic strength of bone in ovariectomized rats. J Nutr 130: 216-220 .
- Leonard F, Boke JW, Ruderman RJ, Hegyeli AF (1972) Initiation and inhibition of subcutaneous calcification. Calcif Tissue Res 10: 269-279 .









Table 1:
Patient characteristics and hemodialysis modality parameters.
n = 45
Age (years)
57.9 ± 17.6
Sex (male/female)
31/14
Time on HD (years)
10.5 ± 8.0
Diabetes mellitus (%)
8.8
CV disease (%)
77.8
HD sessions analyzed in total
13480
HD sessions per patient analyzed
301.8 ± 21.1
HD treatment duration (hrs/week)
4-5
Qb (mL/min)
300
Qd (mL/min)
500
Dialysate HCO3 (mmol/L)
30-36
Dialysate Ca (mmol/L)
1.25