Authors:
Virginia A Aparicio1*, Daniel Camiletti-Moirón1, Mohammed Tassi2, Elena Nebot1, Carlos de-Teresa1,3 and Pilar Aranda1
1Department of Physiology, Faculty of Pharmacy and Institute of Nutrition and Food Technology, University of Granada, Spain
2Department of Pathologic Anatomy and Institute of Regenerative Biomedicine, School of Medicine, University of Granada, Spain
3Andalusian Centre of Sport Medicine, Granada, Spain
Received: 08 June, 2017; Accepted: 24 June, 2017; Published: 26 June, 2017
Virginia A Aparicio, Department of Physiology, Faculty of Pharmacy, Campus de la Cartuja s/n, 18011, Granada, Spain, Tel: 34-958-243882; Fax: 34-958-248959; E-mail:
Aparicio VA, Camiletti-Moirón D, Tassi M, Nebot E, de-Teresa C, et al. (2017) Effects of Anabolic Androgenic Steroids on Renal Morphology in Rats. Arch Renal Dis Manag 3(2): 034-037. DOI: 10.17352/2455-5495.000024
© 2017 Aparicio VA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Corrosive injury; Lye; Acid; Management
Aim: To investigate the long-term effects of anabolic androgenic steroids (AAS) on renal status in rats.
Methods: Twenty Wistar rats were distributed into 2 groups: AAS or placebo, for 3 months. The animal received 10mg/kg body weight of Stanozonol once a week by intramuscular injection in the gluteus, or saline solution as placebo.
Results:Urinary pH was more acidic in the AAS compared to the placebo group (p<0.05). Kidney weight was 15% higher in the AAS compared to the placebo group (p<0.001). Renal glomerular area was 12% higher in the AAS compared to the placebo group (p=0.001). Animals injected with AAS also displayed a no significant but clinically relevant ~20% higher kidney interstitial connective tissue, glomerular tufts and mesangiums.
Conclusions: Overall, AAS negatively affected urinary pH and kidney morphology leading to a worse renal status. More attention should be taken to the use of AAS among populations at high risk for kidney disease.
Introduction
Anabolic androgenic steroids (AAS) are one of the most used performance-enhancing substances among professional athletes as well as recreational body builders [1]. Chronic use of AAS has been known to cause several alterations. Among these disorders, renal diseases have received less attention probably because they are less frequent while heart disease, altered lipid profile and hepatotoxicity have been extensively explored [2,3]. Nephrotoxic side effects have been documented, such as renal failure or Wilms’ tumours [3-7].
As use of AAS is illicit, much of the knowledge of their effects is derived from case reports or retrospective studies. Another limitation of human studies is represented by the fact that information about AAS is generally self-reported, and it is difficult to know the exact dosage. Furthermore, AAS are often used in combination with other substances (e.g. growth hormone) and it is complicated to separate its effects. Consequently, experimental studies conducted in animal models are mandatory given the complexity of carrying out long-term and well-controlled interventional studies in humans. The present study aimed to examine the renal effects of AAS.
Methods
Experimental design
A total of 20 male Wistar rats were allocated into two groups: AAS vs. placebo. The animals, with an initial body weight of 152±8g were housed in group cages. The cages were located in a well-ventilated thermostatically controlled room (21±2°C) with a relative humidity ranging 40-60% and a reverse 12h light-12h dark cycle (08:00-20:00h). Throughout the experimental period (12 weeks) all rats had free access to type 2 water and consumed the diet ad-libitum. Experimental diets were formulated to meet the nutrient requirements of rats based on the AIN-93M formulation.
The rats’ body weights were measured weekly at the same time, and the amount of food consumed by each rat was registered daily.
On week 11, a 12-hour urine sample from each animal was collected for biochemical analysis. At the end of the experimental period, animals were anaesthetized with ketamine-xylazine and sacrificed by cannulation of the abdominal aorta. Blood was collected and centrifuged at 4500rpm to separate plasma that was frozen in liquid N and stored at -80ºC. Carcass weight was recorded. Kidneys were extracted, weighed, and the left one was introduced in formalin for the posterior histological analysis.
All experiments were performed according to the Directional Guides Related to Animal Housing and Care, and all procedures were approved by the Animal Experimentation Ethics Committee of the University of Granada (ref:2011-343).
Anabolic-androgenic steroids
The animals from the experimental group received 10mg/kg body weight of Stanozonol once a week by intramuscular injection in the gluteus (alternating the lateral side). This dosage is comparable to which has been reported as being frequently used by athletes [8]. We used a commercially available Stanozolol solution of 50mg/mL (Winstrol Depot, Zambon) that was diluted to appropriate concentrations and to keep the volume of injection constant. Control groups were injected with saline solution as placebo.
Chemical analyses
Urinary pH was analysed with a bench pH-meter (Crison, Barcelona). Plasma urea, albumin and creatinine concentrations were measured with an autoanalyzer (Hitachi-Roche p800, Hoffmann-La Roche Ltd).
Histological analysis
The left-kidney samples were fixed in 4% buffered formalin and embedded in paraffin. Subsequently, three micrometer sections were cut for a hematoxylin eosin stain and four-micrometer-thick sections were obtained and stained with 1% Picrosirius red F3BA (Gurr, BDH Chemicales Ltd, Poole, United Kingdom) [9]. This technique facilitates the visualization of connective fibers as deep red stains on a pale yellow background [9]. The sections were assessed by optical microscopy. Forty images per sample were captured: 20 of the glomerulus to determine the morphometry and the intraglomerular connective tissue and 20 of the tubulointerstitial area to measure the interstitial connective tissue. All images were acquired using the 20× objective and analyzed with the Fibrosis HR® software [10]. This image analysis application allowed us to automatically quantify morphometric parameters by using various image-processing algorithms.
The following eight morphological variables were stimated: a) Percentage of interstitial connective tissue in reference to the image area, excluding the glomerular area (the connective tissue that is in the gap over the Bowman’s capsule). b) The area of interstitial connective tissue (including Bowman’s capsule). The Fibrosis HR® software divides glomerular tufts into two categories: “glomerular tuft I” and “glomerular tuft II”. The variable “glomerular tuft I” correspond to the renal corpuscle excluding the Bowman’s capsule. The variable “glomerular tuft II” corresponds to the renal corpuscle excluding the Bowman’s capsule and considering the area of the capillary lumens and urinary spaces in the glomerulus. c) Glomerular tuft I area. d) Glomerular tuft II area. e) Glomerular tuft I percentage (percentage of glomerular tuft I related to the glomerular area). f) Glomerular tuft II percentage (percentage of glomerular tuft II related to the glomerular area). g) Mesangial area. h) Glomerular area.
Statistical analysis
Analysis of variance (ANOVA) test was used to compare AAS and placebo groups. Additionally, effect size between groups was calculated by using the Cohen’s d (standardised mean differences) statistic.
All analyses were performed using the Statistical Package for Social Sciences (IBM-SPSS, version 20.0 for Windows), and the level of significance was set at p<0.05.
Results
The effects of AAS on final body weight, carcass weight, food intake, plasma, urinary and renal parameters are shown in table 1. Urinary pH was lower and volume higher in the AAS compared to the placebo group (p<0.05). No differences between groups were observed on body weight, carcass weight, food intake and plasma urea, creatinine and albumin (all, p>0.05).
Kidney weight was ~15% higher in the AAS compared to the placebo group (p=0.009). Renal glomerular area was ~12% higher in the AAS compared to the placebo group (p=0.001). Despite no statistically significant, but with large effect size, AAS group showed ~20% enlarged interstitial connective tissue, glomerular tufts I and II, and mesangiums (Figure 1).
-
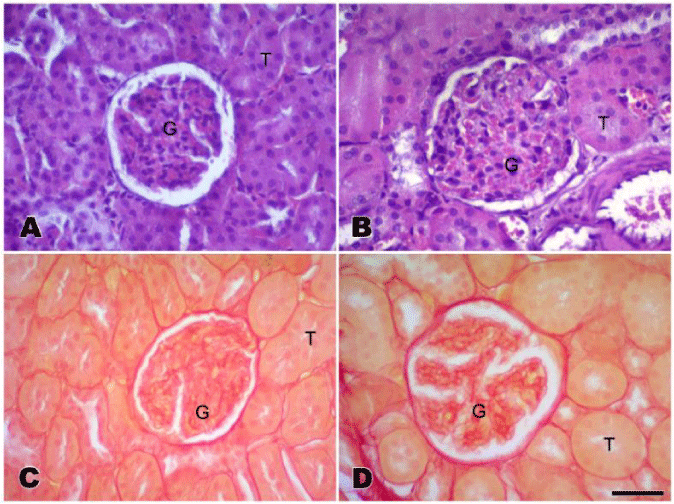
Figure 1:
Histological sections of renal tissue from anabolic androgenic steroids (AAS) group (B, D) and placebo group (A, C). Renal sections were stained with hematoxylin eosin (A, B) to evaluate the general histology and with Picrosirius red (C, D) to study and measure the connective tissue. G: glomerulus. T: renal convoluted tubules. Bar: 50µm.
Discussion
The findings of this study show that AAS induced a worse renal status. AAS increased urinary acidity, kidney weight and renal glomerular area. AAS also clinically worsened the rest of renal morphological variables studied (i.e. ~20% higher interstitial connective tissue, glomerular tufts and mesangiums).
Kidney can be injured in diverse ways by many drugs, both legal and illegal. Novel associations and descriptions of nephrotoxic effects of common and emerging ergogenic drugs have appeared over the past several years [6]. AAS, illicitly used by athletes for decades to increase muscle mass and decrease body fat [1], are emerging as podocyte toxins in long-term abusers [6]. Until date, the effect of AAS on the kidney structure has been scarcely investigated. Acute kidney injuries after AAS abuse usually include focal segmental glomerulosclerosis, glomerulomegaly, tubular atrophy or necrosis, interstitial fibrosis and inflammatory interstitial nephritis [4 5 7]. Oxidative stress may be linked to the pathophysiology of most of these alterations, being involved in fibrosis, cellular proliferation or tumorigenesis [11].
Much of the knowledge of these potentially severe side effects is confounded by the use of combinations of different steroid preparations, or by the concomitant use with other substances [3], whereas we have used a single and controlled AAS administration. To our knowledge, only Hoseini et al. [12] has investigated the morphological renal effects of AAS in a controlled animal model like ours. The treated group received 3mg/kg of body weight of Nandrolone decanoate intraperitoneally administered in one, two and three doses, respectively, in the first, second and third week of treatment. In agreement to our data, kidney weight increased by 30% and its volume by 25%. They also observed greater volume of the cortex, proximal and distal convoluted tubules. In contrast to our results, they did not find increases in the glomerulus volume [12].
Overall, the present physiological and morphological findings obtained in rodents must be confirmed in humans. However, studies analysing the effects of AAS on renal status are scarce or inexistent. Moreover, this study explored, in a well-controlled experimental model, the renal effects of AAS in the long time.
Funding
This study was supported by the project DEP2008-04376 from the Spanish Ministry of Science and Innovation. VAA was also supported by the Andalucía Talent Hub Program launched by the Andalusian Knowledge Agency, co-funded by the European Union’s Seventh Framework Program, Marie Skłodowska-Curie actions (COFUND–Grant Agreement nº291780) and the Ministry of Economy, Innovation, Science and Employment of the Junta de Andalucía.
- Sjoqvist F, Garle M, Rane A (2008) Use of doping agents, particularly anabolic steroids, in sports and society. Lancet 371: 1872-1882. Link: https://goo.gl/MEPHTd
- Bonetti A, Tirelli F, Catapano A (2008) Side effects of anabolic androgenic steroids abuse. Int J Sports Med 29: 679-687. Link: https://goo.gl/UcFYNM
- Modlinski R, Fields KB (2006) the effect of anabolic steroids on the gastrointestinal system, kidneys, and adrenal glands. Curr Sports Med Rep 5: 104-109. Link: https://goo.gl/Ltg3ga
- Herlitz LC, Markowitz GS, Farris AB, Joshua AS, Michael BS, et al. (2010) Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163-172. Link: https://goo.gl/fCvZn1
- Daher EF, Silva Junior GB, Queiroz AL, Lysiane MA Ramos, Silvia QS, et al. (2009) acute kidney injury due to anabolic steroid and vitamin supplement abuse: report of two cases and a literature review. Int Urol Nephrol 41: 717-723. Link: https://goo.gl/12CJNJ
- Pendergraft WF, 3rd, Herlitz LC, Thornley-Brown D, Mitchell R, John LN, et al. (2014) Nephrotoxic Effects of Common and Emerging Drugs of Abuse. Clinical journal of the American Society of Nephrology: CJASN 9: 1996-2005. Link: https://goo.gl/Cs67pa
- Almukhtar SE, Abbas AA, Muhealdeen DN, Michael DH (2015) Acute kidney injury associated with androgenic steroids and nutritional supplements in bodybuilders(dagger). Clinical kidney journal 8: 415-9. Link: https://goo.gl/2W7m77
- Ip EJ, Barnett MJ, Tenerowicz MJ, Paul Jp (2011) The Anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy 31: 757-766. Link: https://goo.gl/96AZfy
- Sweat F, Puchtler H (1964) Rosenthal SI. Sirius red f3ba as a stain for connective tissue. Arch Pathol 78: 69-72. Link: https://goo.gl/uUmMu3
- Masseroli M, O'Valle F, Andujar M, Ramírez C, Gómez MM, et al. (1998) Design and validation of a new image analysis method for automatic quantification of interstitial fibrosis and glomerular morphometry. Lab Invest 78: 511-522. Link: https://goo.gl/zNFkoJ
- Frankenfeld SP, Oliveira LP, Ortenzi VH, Victor HO, Igor CC Rego Monteiro, et al. (2014) The anabolic androgenic steroid nandrolone decanoate disrupts redox homeostasis in liver, heart and kidney of male Wistar rats. Link: https://goo.gl/7hNNYA
- Hoseini L, Roozbeh J, Sagheb M, Saied KD, Ali N (2009) Nandrolone decanoate increases the volume but not the length of the proximal and distal convoluted tubules of the mouse kidney. Micron 40: 226-230. Link: https://goo.gl/cV8M2j









Table 1:
Effects of anabolic androgenic steroids (AAS) on plasma and urinary markers and kidney morphology.
Variable
AAS
Placebo
P
Effect size †
Final body weight (g)
340.7 (36.8)
345.4 (31.5)
0.675
-0.14 (-1.06, 0.79)
Carcass weight (g)
180.0 (15.4)
172.5 (19.6)
0.066
0.42 (-0.51,1,36)
Food intake (g/day)
17.96 (2.6)
17.65 (1.8)
0.660
0.58 (-0.36, 1.52)
Kidney (g) (mean right and left)
1.07 (0.13)
0.91 (0.10)
<0.001
1.37 (0.35, 2.41)
Kidney (g/100g body weight)
0.32 (0.04)
0.26 (0.03)
<0.001
1.70 (0.61, 2.78)
Kidney (g/100g carcass weight)
0.61 (0.06)
0.54 (0.04)
0.009
1.37 (0.34, 2.40)
Plasma
Urea (mg/dL)
27.6 (3.49)
25.0 (5.13)
0.202
0.59 (-0.35, 1.53)
Albumin (mg/dL)
3.02 (0.63)
2.73 (0.84)
0.296
0.40 (-0.54, 1.32)
Creatinine (mg/dL)
0.42 (0.08)
0.49 (0.18)
0.231
-0.50 (-1.44, 0.43)
Urine
Urinary pH
6.83 (0.54)
7.00 (0.50)
0.024
-0.33 (-1.26, 0.60)
Urinary volume (mL)
4.37 (2.54)
2.86 (0.96)
0.023
0.80 (-0.17, 1.76)
Kidney morphology
Interstitial connective tissue (%)
3.28 (1.48)
2.71 (1.25)
0.366
0.42 (-0.52, 1.35)
Interstitial connective tissue area (µm²)
4213 (1890)
3656 (1734)
0.295
0.31 (-0.63, 1.24)
Glomerular tuft I (%)
20.5 (8.3)
16.7 (7.50)
0.152
0.48 (-0.45, 1.42)
Glomerular tuft I area (µm²)
8542 (4425)
6616 (2652)
0.247
0.52 (-0.41, 1.47)
Glomerular tuft II (%)
45.1 (11.9)
37.1 (16.3)
0.162
0.56 (-0.38, 1.51)
Glomerular tuft II area (µm²)
17809 (8125)
14573 (5871)
0.168
0.45 (-0.48, 1.40)
Mesangium area (µm²)
5336 (2675)
4172 (1501)
0.717
0.53 (-0.40, 1.48)
Mesangium percentage (%)
65.6 (8.9)
64.7 (3.94)
0.440
0.13 (-0.80, 1.06)
Glomerular area (µm²)
45629 (4034)
40404 (3354)
0.001
1.41 (0.37, 2.44)