Authors:
Marvin Rubenstein1-4*, Courtney M.P. Hollowell4 and Patrick Guinan1,3,4,5
1Division of Cellular Biology, Hektoen Institute for Medical Research, Chicago, IL, 60612, USA
2Departments of Urology Rush University Medical Center, Chicago, IL, 60612, USA
3Biochemistry and Urology Rush University Medical Center, Chicago, IL, 60612, USA
4Division of Urology, Stroger Hospital of Cook County, Chicago, IL, 60612, USA
5Department of Urology, University of Illinois at Chicago, Chicago, IL, 60612, USA
Received: 26 February, 2014; Accepted: 25 March, 2014; Published: 27 March, 2014
Dr. Marvin Rubenstein, Chairman, Division of Cellular Biology, Hektoen Institute for Medical Research, 2240 West Ogden Avenue, Chicago, IL, 60612, USA, Tel: +1 312 864 4621; Fax: +1 312 768 6010; Email: DrMarv@Prodigy.net
Rubenstein M, Hollowell CMP, Guinan P (2015) In LNCaP Cells Inhibition of BCL-2 by Antisense Oligonucleonucleotides Results in Compensatory Changes in Apoptosis. Scientific J Genetics Gen Ther 1(1): 001-006.
© 2015 Rubenstein M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Antisense oligonucleotides; Prostate cancer; BCL-2; bax; caspase-3; clusterin; AKT-1; Therapy
Antisense oligonucleotides (oligos) have been evaluated for treating prostate cancer in both in vivo and in vitro models. Although most oligos contain a single mRNA binding site, our laboratory evaluates bi-specific oligos directed towards two proteins. This study evaluates the growth inhibition in vitro of the LNCaP cell line employing mono- and bi-specific oligos directed against BCL-2 [the second binding site was directed against the epidermal growth factor receptor (EGFR)]. These oligos were administered with lipofectin as part of a nanoparticle delivery system. Additionally, g, the expression of five apoptosis regulatory proteins, BCL-2, bax, caspase-3, clusterin and AKT-1 was evaluated by RT-PCR.
LNCaP prostate tumor cells were incubated in the presence of oligos specifically directed against BCL-2 and their effect was compared to lipofectin containing controls. Significant, but comparable, growth inhibition was produced by both mono- and bi-specific forms. Employing RT-PCR to determine BCL-2 expression, we found that the greatest amount of mRNA suppression approached 100% for each type of oligo with mono-specific MR4 (directed only against BCL-2) equal 100%; and bispecifics MR24 and MR42, being 86% and 100% respectively. Based upon both inhibition of cell growth and BCL-2 expression, bi-specific antisense oligos directed against EGFR and BCL-2 mRNAs are at least as effective as a mono-specific directed solely towards BCL-2.
In an effort to determine a compensatory response by cells needed to evade apoptosis in the presence of BCL-2 suppression, the levels of mRNA encoding non-targeted bax, caspase-3, clusterin and AKT-1 were evaluated. Suppression of the apoptosis inhibitor (BCL-2) in LNCaP cells did not alter either bax or clusterin expression. However, the expression of non-targeted caspase-3 (an apoptosis promoter) was suppressed and the expression of non-targeted AKT-1 (an apoptosis inhibitor) was enhanced. This suggests that tumor variants can resist apoptosis through the altered expression of non-targeted regulators of apoptosis. If BCL-2 suppression was to be clinically targeted by antisense oligos, similar experiments may be helpful in identifying genes with a similar function.
Introduction
Antisense oligonucleotides (oligos) have been evaluated in both in vivo and in vitro prostate cancer models primarily through suppression of protein growth factors, androgens (e.g., oligos directed against 5-alpha-reductase), receptors which bind these factors, regulators of apoptosis and oncogenes. Those which have advanced to clinical trials focus on the inhibitors of apoptosis (BCL-2 [directly an inhibitor] and clusterin [an inhibitor of activated bax, which is a promoter]). Oligos provide a specific, relatively non-toxic method for translational arrest, and almost all are directed against single gene products or those which shared sequence homology. Since most tumors are characterized by the enhanced expression of many proteins, tumor growth is unlikely to be inhibited by knockdown of single gene products. Instead, multiple genes must be downregulated. Therefore, our laboratory evaluates bi-specific oligos which target two distinct proteins (which are able to regulate different pathways and do not share sequence homology). These bi-specifics differ from those targeting genes which share sequence homology (BCL-2 and BCL-xL) [1Yamanaka K, Miyake H, Zangemeister-wittke U, Jansen B, Gleave M (2004) Novel bispecific antisense oligonucleotides inhibiting both BCL-2 and Bcl-xL expression induce apoptosis and enhance chemosensitivity in human androgen-independent prostate cancer cells. Proceedings AACR 45.] or the OGX-225 oligo which targets (three) structurally related insulin like growth factor binding proteins [2Takahara K, Muramaki M, Li D, Cox ME, Gleave ME (2009) Insulin-like growth factor-binding protein-5 (IGFBP5) supports prostate cancer cell proliferation. J Urol 181.]. We have demonstrated that the addition of a second binding site does not affect the activity of the first and that dual binding sites can simultaneously be directed against genes involved in either a single growth promoting autocrine loop [3Rubenstein M, Tsui P, Guinan P (2006) Bispecific antisense oligonucleotides with multiple binding sites for the treatment of prostate tumors and their applicability to combination therapy. Meth Find Clin Pharmacol 28: 515-518.] or towards those of even different regulatory pathways [4Rubenstein M, Tsui P, Guinan P (2007) Combination chemotherapy employing bispecific antisense oligonucleotides having binding sites directed against an autocrine regulated growth pathway and BCL-2 for the treatment of prostate cancer. Med Oncol 24: 372-378.-7Rubenstein M, Tsui P, Guinan P (2008) Treatment of MCF-7 breast cancer cells employing mono- and bispecific antisense oligonucleotides having binding specificity toward proteins associated with autocrine regulated growth and BCL-2. Med Oncol 25: 182-186. ]. While most assessments of activity quantitate the inhibition of in vitro cell growth, more specific methods use the polymerase chain reaction (PCR) to measure specific protein encoding mRNA.
Previously we have shown that in LNCaP cells a single mono- and two bispecific oligos directed towards BCL-2 increase the expression of the androgen receptor (AR) [8Rubenstein M, Hollowell CMP, Guinan P (2011) In LNCaP cells enhanced expression of the androgen receptor compensates for bcl-2 suppression by antisense oligonucleotides. Ther Adv Urol 3: 73-79. ] and its co-activating transcription factors p300 and interleukin-6 (IL-6) [9Rubenstein M, Hollowell CMP, Guinan P (2013) Increased expression of the androgen receptor with p300 and IL-6 coactivators compensate for oligonucleotide suppression of bcl-2. No increased CREB binding protein or IL-4 expression. Ther Adv Urol 5: 85-93. ]. Since this pattern of gene expression is often associated with later stage tumors, we postulated that suppressive therapy against BCL-2 could lead to increased androgen sensitivity and promote tumor aggressiveness. It is likely that additional compensatory mechanisms exist, and since regulation of apoptosis is clinically receptive to oligo treatment, we evaluated several non-targeted proteins of this process to identify other proteins associated with compensation and apoptosis evasion. We used the same mono- and bi-specific oligos as in the androgen sensitivity studies and RT-PCR to evaluate BCL-2, bax, caspase-3, clusterin and AKT-1. These proteins were chosen due to their ability to regulate apoptosis in two opposing manners; BCL-2, clusterin and AKT-1 are inhibitory, while activated bax and caspase-3 stimulate the process.
If gene therapy is to be effective when directed against BCL-2, compensatory changes which compromise the effectiveness of the desired suppression must be identified. Among a mass of heterogeneous tumor cells, those which evade apoptosis are the most likely to be selected. Altered patterns of gene expression could include increased inhibition by BCL-2, clusterin or AKT-1 or decreased promotion by bax and caspase-3. If compensatory changes are initiated by therapy resulting in BCL-2 suppression it is important to identify those gene activities which must either be maintained or replaced. Such modification would make BCL-2 directed gene therapy more efficacious, as suggested in the LNCaP prostate cancer model. Nanoparticle delivery systems are particularly effective to promote oligo entry into targeted cells, and these methods should be further explored to enhance gene therapy.
Methods
Oligonucleotides
Oligos (mono- or bispecific) were purchased from Eurofins MWG Operon (Huntsville, AL, USA). Each was phosphorothioated on three terminal bases at 5’ and 3’ positions. Stock solutions were made to a final concentration of 625 μM in sterile Dulbecco phosphate buffered saline (PBS).
Base sequences
Each oligo contained at least one CAT sequence and targeted the area adjacent to the AUG initiation codon for mRNA encoding the respective targeted protein (BCL-2 or EGFR).
MR4 (mono-specific targeting BCL-2): T-C-T-C-C-C-A-G-C-G-T-G-C-G-C-C-A-T
MR24 (bi-specific targeting EGFR/BCL-2): G-A-G-G-G-T-C-G-C-A-T-C-G-C-T-G-C-T-C- T-C-T-C-C-C-A-G-C-G-T-G-C-G-C-C-A-T
MR42 (bi-specific targeting BCL-2/EGFR): T-C-T-C-C-C-A-G-C-G-T-G-C-G-C-C-A-T-G-A-G-G-G-T-C-G-C-A-T-C-G-C-T-G-C-T-C
Cell culture
LNCaP cells (American Type Culture Collection, Manassas, VA, USA) were grown in RPMI 1640 supplemented with 10% bovine serum, 1% L-glutamine and 1% penicillin/streptomycin in a 5% CO2 incubator. Log phase cells were harvested using EDTA/Trypsin and equally distributed into 75 cm2 flasks (Corning, NY, USA).
Determination of growth
Four days prior to the addition of oligos 1 x 104 LNCaP cells were added, in a total 200 μl volume of media, to each depression of a 96-well plate and incubated at 37o C in a 5% incubator [3Rubenstein M, Tsui P, Guinan P (2006) Bispecific antisense oligonucleotides with multiple binding sites for the treatment of prostate tumors and their applicability to combination therapy. Meth Find Clin Pharmacol 28: 515-518.].
Cells were incubated for 24-48 hrs before solutions were aspirated and re-incubated for an additional 48 hrs in 200 μl of media. Cell counts were determined following the addition of WST-1 reagent to each well, and after 2 hrs the color intensity was measured by a microplate reader at a wavelength of 450 nm, using a reference of 650 nm. Values obtained were determined after the subtraction of paired blank samples from the experimental wells and were multiplied by a constant to give whole integers for analysis. Microsoft Excel software was utilized to calculate means and standard deviations, and Students t tests were used to determine significance.
Oligo treatment prior to PCR
Fours days prior to oligo addition, when cell density approached 75% confluence, 10 mL of fresh media was added. Cells were incubated for an additional 3 d before 5 mL of media was replaced with fresh the day before oligos were added. 100 μl of stock oligos were added to bring the final concentration to 6.25 μM. Incubation proceeded for an additional 24 hrs in the presence or absence of mono-specific MR4, or the MR24 and MR42 bi-specific oligos.
RNA extraction
Following treatment, media was removed, and 1 mL of cold (4oC) RNAzol B was added to each 75 cm2 culture flask and the monolayer lysed by repeated passage through a pipette. All procedures were performed at 4oC. The lysate was removed, placed in a centrifuge tube to which 0.2 ml of chloroform was added, and shaken. The mixture stayed on ice for 5 min, was spun at 12,000 x g for 15 min, and the upper aqueous volume removed and placed in a fresh tube. An equal volume of isopropanol was added, the tube shaken, and then allowed to stay at 4oC for 15 min before similar centrifugation to pellet the RNA. The supernatant was removed, the pellet washed in a single ml of 75% ethanol, then spun for 8 min at 7500 x g. The ethanol was pipetted off and the formed pellet air dried at -20oC.
RNA quantitation
RNA was resuspended in 250 μL of diethylpyrocarbonate (DEPC) treated water (Invitrogen), and quantitated using a Qubit florometer and Quant-iT RNA assay kit (Invitrogen). DEPC is an inhibitor of RNase activity.
RT-PCR
Extracted RNA was diluted to 40 μg/μL in DEPC treated water. 1 μL of this RNA was added to1 μL of both sense and antisense primers (forward and reverse sequences from Invitrogen) for human actin (used as a control) or 2 μL of combined primers for BCL-2, bax, caspase-3, clusterin or AKT-1 (RealTimePrimers, Elkins Park, PA). Primers were provided in 50 μM solution of Tris-HCl (pH 7.5, 0.1 mM EDTA. From a kit purchased from Invitrogen the following reactants were added for RT-PCR: 25 μL of 2x reaction mixture, 2 μL SuperScript III RT/platinum Taq mix, tracking dye, and MgSO4 (3 μL of a stock concentration of 5mM, used for BCL-2, bax, caspase-3, clusterin and AKT-1 vials only). DEPC treated water was added to yield a final volume of 50 μL. As a standard control for RT-PCR product production, human actin expression was tested in RNA extracted from HeLa cells which was provided in a kit purchased from Invitrogen . RT-PCR was performed for 2 x 25 cycles using the F54 program in a Sprint PCR Thermocycler.
Primers
Actin:
Forward primer sequence: 5’ CAA ACA TGA TCT GGG TCA TCT TCT C 3’
Reverse primer sequence: 5’ GCT CGT CGT CGA CAA CGG CTC
PCR product produced was 353 base pairs in length.
BCL-2
Forward primer sequence: 5’ GAG ACA GCC AGG AGA AAT CA 3’
Reverse primer sequence: 5’ CCT GTG GAT GAC TGA GTA CC 3’
PCR product produced was 127 base pairs in length.
Bax
Forward primer sequence: 5’ GCT GGA CAT TGG ACT TCC TC 3’
Reverse primer sequence: 5’ CTC AGC CCA TCT TCT TCC AG 3’
PCR product produced was 168 base pairs in length.
Caspase-3
Forward primer sequence: 5’ CCC CTG GAT CTA CCA GCA TA 3’
Reverse primer sequence: 5’ TGT CTC TGC TCA GGC TCA AA 3’
PCR product produced was 262 base pairs in length.
Clusterin
Forward primer sequence: 5’ GGA GGA GTG AGA TGT GGA TG 3’
Reverse primer sequence: 5’ ATG CAG GAG CAA TTC TGT TC 3’
PCR product produced was 221 base pairs in length.
AKT-1
Forward primer sequence: 5’ ACC TTT TCG ACG CTT AAC CT 3’
Reverse primer sequence: 5’ TGG AGG GAA GGT TCC ATA TT 3’
PCR product produced was 189 base pairs in length.
Detection and quantitation of product
Agarose gel electrophoresis: 1.5% agarose gels were prepared in a 50 mL volume of TBE buffer (1x solution: 0.089 M Tris borate and 0.002M EDTA, pH 8.3), containing 3 μl of ethidium bromide (10 mg/mL in 1x Tris borate buffer) in a Fisher Biotest electrophoresis system. Samples were run for 2 hrs at a constant voltage of 70 using a BioRad 1000/500 power supply source. To locate the amplified PCR product, 3 μL of a molecular marker which contained a sequence of bases in 100 base pair increments (Invitrogen) as well as 2 μL of a sucrose based bromphenol blue tracking dye were run in each gel. For actin product localization, the tracking dye was included in each sample run; for all other products the tracking dye was run separately.
Quantitation: Gels were visualized under UV light and photographed using a Canon PowerShot Elph300HS digital camera. Photographs were converted to black and white format and bands quantitated using Mipav software provided by the National Institute of Health (NIH).
Results
Cell culture experiments
LNCaP cells were incubated with MR4, MR24 and MR42 and compared to lipofectin-treated controls (Figure 1). In an initial experiment each oligo significantly inhibited the growth of LNCaP cells: MR4 by 23.8% (P = 0.0004); MR24 by 31.2% (P < 0.001); and MR42 by 31.7% (P < 0.001).
In a repeat experiment LNCaP cells were similarly incubated and compared to lipofectin containing controls. Bi-specific oligos MR24, and MR42 produced significant respective inhibitions of 49.5% (P < 0.001) and 56.8% (P < 0.001), and were at least as effective as the mono-specific MR4 directed only towards BCL-2 in the inhibition of in vitro cell growth. Dosage was determined based upon previous experience employing monospecific oligos and remained consistent with conditions described in US patents #5,891,858 and 5,610,288.
RT-PCR experiments
Actin Control: As a RT-PCR control the expression of actin was employed using RNA obtained from untreated LNCaP cells. Figure 2 provides an example of one such band (actin) suitable for scanning and quantitation . The molecular weight markers shown in the left column are (in 100 base pair increments) 600 and 100 base pairs (from top to bottom). The band visualized between molecular markers of 300 and 400 base pairs is the expected 353 base pair human actin PCR product.
BCL-2 expression
LNCaP cells incubated for 24 hours in the presence of 6.25 μM of oligos demonstrated a suppression of BCL-2 expression, and support the finding of comparable biologic activity of both mono- and bi-specific oligos seen in in vitro cell inhibition experiments. For each oligo evaluated, the greatest amount of suppression measured approached 100%, for the mono-specific MR4; and for the MR24 and MR42 bi-specifics, 86 and 100%, respectively. Suppression was found in both repeat PCR runs with BCL-2 primers, as well as in repetitive agarose gel quantifications. Figure 3 is a representative gel which presents a BCL-2 product band in the expected 127 base pair region. This run was inhibited 23% by treatment with the mono-specific MR4, and 86 and 74%, respectively by the MR24 and MR42 bi-specifics, as measured by Mipav software. As mentioned above, other gels evaluated exhibited suppression approaching 100% (This gel was chosen because it contained visible bands which were not totally suppressed and can be visualized within the expected base pair position).
-

Figure 3
Suppressed Expression of BCL-2 on Agarose Gel. For each oligo evaluated, the greatest amount of suppression measured approached 100%, for the mono-specific MR4; and for the MR24 and MR42 bi-specifics, 86 and 100%, respectively. In this representative gel, which is able to show suppressed bands, there was 23% inhibition by treatment with the mono-specific MR4, and 86 and 74%, respectively by the MR24 and MR42 bi-specifics.
Based upon both inhibition of cell growth and BCL-2 expression we conclude that bi-specific antisense oligos directed against EGFR and BCL-2, regardless of their tandem orientation, are at least as effective as the mono specific type directed solely towards BCL-2. The addition of a second mRNA binding site (directed to EGFR) on these oligos does not prevent activity at the site specific for BCL-2 [3Rubenstein M, Tsui P, Guinan P (2006) Bispecific antisense oligonucleotides with multiple binding sites for the treatment of prostate tumors and their applicability to combination therapy. Meth Find Clin Pharmacol 28: 515-518.].
Bax expression
Identical amounts of extracted RNA from LNCaP cells treated with either mono- or bi-specific oligos directed against BCL-2 (and EGFR in the bispecifics) were evaluated by RT-PCR using primers directed against bax. A representative band for bax is presented in Figure 4 and appears immediately below the marker representing 200 base pairs .
-

Figure 4
Bax Expression is Unaffected by Oligos as Indicated in Agarose Gel. Presented above is a representative gel. The average relative intensity of the bands corresponding to bax representing cells treated with MR4, MR24 and MR42 compared to controls were -5.74 ± 16.9, 5.54 ±19.2, and -15.34 ± 32.9 indicating no significant differences in bax expression.
When background intensity was subtracted, the relative intensity of the bands corresponding to bax representing cells treated with MR4, MR24 and MR42 compared to controls were -5.74 ± 16.9, 5.54 ±19.2, and -15.34 ± 32.9. These results were pooled from both duplicate PCR runs and gels and indicated that no significant differences in bax expression were found, compared to that seen with BCL-2.
Caspase-3 expression
Identical amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against BCL-2 (and EGFR in the bispecifics) was evaluated by RT-PCR using primers directed against caspase-3. A representative band for caspase-3 is presented in Figure 5 and appears immediately below the marker representing 300 base pairs.
-

Figure 5
Decreased Expression of Caspase-3 in Agarose Gel. Presented above is a representative gel. The average relative intensity of the bands corresponding to caspase-3 representing cells treated with MR4, MR24 and MR42 compared to controls were -35.8 ± 12.5 (P = 0.0002), -40.3 ± 16.6 P = 0.0006) and -43.5 ± 26.3 (P = 0.006) indicating that the expression of caspase-3 is significantly enhanced by each oligo type.
When background intensity was subtracted, the relative intensity of the bands corresponding to caspase-3 representing cells treated with MR4, MR24 and MR42 compared to controls were -35.8 ± 12.5 (P = 0.0002), -40.3 ± 16.6 P = 0.0006) and -43.5 ± 26.3 (P = 0.006). These results were pooled from both duplicate PCR runs and gels and indicated that similar significant suppression of caspase-3 expression was demonstrated with each oligo (and type) evaluated. This result would suggest that when inhibiting BCL-2, caspase-3 activity should be either maintained or replaced.
Clusterin expression
Comparable amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against BCL-2 (and EGFR in the bispecifics) was then evaluated by RT-PCR using primers directed against clusterin. A representative band for clusterin is presented in Figure 6 and appears where expected between the markers representing 200 and 300 base pairs, as a 221 base pair product .
-

Figure 6
Clusterin Expression is Unaffected by Oligos as Indicated in Agarose Gel. Presented above is a representative gel. The average relative intensity of the bands corresponding to clusterin representing cells treated with MR4, MR24 and MR42 compared to controls were 8.3% ± 14.5, 9.0% ±17.3, and -14.1% ± 22.6 indicating no significant differences in clusterin expression.
When background intensity was subtracted, the relative intensity of the bands corresponding to clusterin representing cells treated with MR4, MR24 and MR42 compared to controls were 8.3% ± 14.5, 9.0% ±17.3, and -14.1% ± 22.6 (mean ± SD). These results were pooled from both duplicate PCR runs and gels, indicating (like bax) there are no significant differences in clusterin expression, compared to that seen with caspase-3.
AKT-1 expression
Identical amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against BCL-2 (and EGFR in the bispecifics) was then evaluated by RT-PCR using primers directed against AKT-1. A representative band 1 is presented in Figure 7 and appears as a 189 base pair product.
When background intensity was subtracted, the relative intensity of the bands corresponding to AKT-1 representing cells treated with MR4, MR24 and MR42 compared to controls were increased 256.7% ± 105.5 (P = 0.0006), 189.4% ±73.6 (P = 0.0004), and 182.6% ± 90.8 (P = 0.002) (mean ± SD). These results indicate that the expression of the apoptotic inhibitory protein AKT-1 is enhanced, identifying another compensatory response to BCL-2 suppression.
-

Figure 7
Increased Expression of AKT-1 in Agarose Gel. Presented above is a representative gel. The average relative intensity of the bands corresponding to AKT-1 representing cells treated with MR4, MR24 and MR42 were increased 256.7% ± 105.5 (P = 0.0006), 189.4% ±73.6 (P = 0.0004), and 182.6% ± 90.8 (P = 0.002) indicating the expression of AKT-1 is significantly enhanced by each oligo type.
Discussion
Gene therapy for cancer presents a far more complex challenge than treating single gene inherited deficiencies because for it to work numerous pathways (and many of their regulatory proteins) must be simultaneously regulated (suppressed or replaced). Potentially hundreds or even thousands of genes may be involved or having altered expression patterns. The challenge is to identify those gene products which are critical to either continuing the process of malignant transformation or those which maintain normal differentiated function.
For gene products which are overexpressed, methods to suppress either their translation or activities have been developed, including the use of antisense oligos. For the treatment of prostate cancer, some (produced by Oncogenex Pharmaceuticals) have reached clinical trials (OGX-011), while others continue their preclinical development (OGX-225). Often administered in combination with traditional chemotherapy, these oligos target proteins which include BCL-2, clusterin (OGX-011 in Phase II testing), heat shock protein 27 (OGX-427) and insulin growth factor binding proteins (OGX-225). The most promising approach involves efforts to restore tumor apoptosis by eliminating proteins associated with this aspect of tumor resistance.
For those proteins diminished or lacking in expression gene replacement, promotion or amplification would be necessary. Although not as far advanced as gene suppressive therapy, suppressor gene PTEN has been replaced through adenoviral transfection in a non-small cell lung cancer model, where it restored effective radiation treatment through diminished capacity for DNA repair [10Pappas G, Zumstein LA, Munshi A, Hobbs M, Meyn RE (2007) Adenoviral-mediated PTEN expression radiosensitizes non-small cell lung cancer cell by suppressing DNA repair capacity. Gene Therapy 14: 543-549.]. In a prostate cancer model PTEN activity is associated with suppression of BCL-2 and increased chemosensitivity [11Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, et al. (2001) PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of BCL-2 expression. J Biol Chem 276: 38830-388306.]. In addition, antisense treatment directed against BCL-2 increases radiosensitivity in both prostate [12Yip KW, Mocanu JD, Au PY, Sleep GT, Busson D, et al. (2005) Combination BCL-2 antisense and radiation therapy for nasopharyngeal cancer. Clin. Cancer Res 11: 8131-8144.] and nasopharyngeal [13 Mu Z, Hachem P, Pollack A (2005) Antisense BCL-2 sensitizes prostate cancer cells to radiation. The Prostate 65: 331-340. ] cancers.
Tumor cells are heterogeneous and those which evade growth regulation or apoptosis are positively selected. In addition, variants, as their DNA becomes increasingly unstable, tend to accumulate additional adaptations (mutations) which further contribute to resistance and dissemination.
Treatment protocols administered to correct one genetic alteration can, through selective pressure, initiate compensatory changes which diminish the effectiveness of the original, and in many tumors, some of the early mutational events lead to evasion of apoptosis. This “programmed” process clears the body of altered (transformed) or damaged cells. It is highly regulated and involves many proteins being synthesized, recognized (via receptors) or otherwise interacting with each other. Selection of cells which resist apoptosis is no different than the process by which hormone sensitive prostate tumor cells, in the absence of androgen, are selected (and establish themselves) as insensitive variants. Therefore, for suppressive gene therapy to work, it’s important to identify compensatory effects.
Previous work identified increased androgen sensitivity as one mechanism compensating for BCL-2 suppression. In this study we evaluated the effect of oligo mediated growth suppression on various inhibitors (BCL-2, clusterin, AKT-1) and promoters (bax, caspase-3) of apoptosis and found that within this pathway at least two proteins (caspase-3 and AKT-1) compensate for BCL-2 suppression.
We conclude that this study identifies a new form of tumor resistance which results in a compensatory evasion of apoptosis. Together with the previously identified increased androgen sensitivity which produces a gene expression pattern often seen in later stage tumors, suppressive BCL-2 directed therapy could result not only in restored apoptosis, but increased androgen stimulation and tumor aggressiveness.
For gene therapy to be successful, additional effects on untargeted genes must be identified. This is particularly important when translational inhibitors are administered and directed against BCL-2 (particularly for the treatment of prostate cancer). Should additional proteins (inhibitors of apoptosis) be indicated for suppression, bispecific oligos or even proposed multifunctional and branched derivatives could then be employed [14Rubenstein M, Anderson KM, Tsui P, Guinan P (2006) Synthesis of branched antisense oligonucleotides having multiple specificities. Treatment of hormone insensitive prostate cancer. Med Hypoth 67: 1375-1380.]. Additional studies are underway to identify altered expressions in other proteins associated with apoptosis.
Acknowledgments
The Cellular Biology laboratory at the Hektoen Institute is supported, in part, by the Blum Kovler Foundation, the Cancer Federation, Safeway/Dominicks Campaign for Breast Cancer Awareness, Lawn Manor Beth Jacob Hebrew Congregation, the Max Goldenberg Foundation, the Sternfeld Family Foundation, and the Herbert C. Wenske Foundation.
- Yamanaka K, Miyake H, Zangemeister-wittke U, Jansen B, Gleave M (2004) Novel bispecific antisense oligonucleotides inhibiting both BCL-2 and Bcl-xL expression induce apoptosis and enhance chemosensitivity in human androgen-independent prostate cancer cells. Proceedings AACR 45.
- Takahara K, Muramaki M, Li D, Cox ME, Gleave ME (2009) Insulin-like growth factor-binding protein-5 (IGFBP5) supports prostate cancer cell proliferation. J Urol 181.
- Rubenstein M, Tsui P, Guinan P (2006) Bispecific antisense oligonucleotides with multiple binding sites for the treatment of prostate tumors and their applicability to combination therapy. Meth Find Clin Pharmacol 28: 515-518.
- Rubenstein M, Tsui P, Guinan P (2007) Combination chemotherapy employing bispecific antisense oligonucleotides having binding sites directed against an autocrine regulated growth pathway and BCL-2 for the treatment of prostate cancer. Med Oncol 24: 372-378.
- Rubenstein M, Tsui P, Guinan P (2009) Multigene targeting of signal transduction pathways for the treatment of breast and prostate tumors. Comparison between combination therapies employing bispecific oligonucleotides with either Rapamycin or Paclitaxel. Med Oncol 26: 124-130.
- Rubenstein M, Tsui P, Guinan P (2007) Bispecific antisense oligonucleotides having binding sites directed against an autocrine regulated growth pathway and BCL-2 for the treatment of prostate tumors. Med Oncol 24: 189-196.
- Rubenstein M, Tsui P, Guinan P (2008) Treatment of MCF-7 breast cancer cells employing mono- and bispecific antisense oligonucleotides having binding specificity toward proteins associated with autocrine regulated growth and BCL-2. Med Oncol 25: 182-186.
- Rubenstein M, Hollowell CMP, Guinan P (2011) In LNCaP cells enhanced expression of the androgen receptor compensates for bcl-2 suppression by antisense oligonucleotides. Ther Adv Urol 3: 73-79.
- Rubenstein M, Hollowell CMP, Guinan P (2013) Increased expression of the androgen receptor with p300 and IL-6 coactivators compensate for oligonucleotide suppression of bcl-2. No increased CREB binding protein or IL-4 expression. Ther Adv Urol 5: 85-93.
- Pappas G, Zumstein LA, Munshi A, Hobbs M, Meyn RE (2007) Adenoviral-mediated PTEN expression radiosensitizes non-small cell lung cancer cell by suppressing DNA repair capacity. Gene Therapy 14: 543-549.
- Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, et al. (2001) PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of BCL-2 expression. J Biol Chem 276: 38830-388306.
- Yip KW, Mocanu JD, Au PY, Sleep GT, Busson D, et al. (2005) Combination BCL-2 antisense and radiation therapy for nasopharyngeal cancer. Clin. Cancer Res 11: 8131-8144.
- Mu Z, Hachem P, Pollack A (2005) Antisense BCL-2 sensitizes prostate cancer cells to radiation. The Prostate 65: 331-340.
- Rubenstein M, Anderson KM, Tsui P, Guinan P (2006) Synthesis of branched antisense oligonucleotides having multiple specificities. Treatment of hormone insensitive prostate cancer. Med Hypoth 67: 1375-1380.




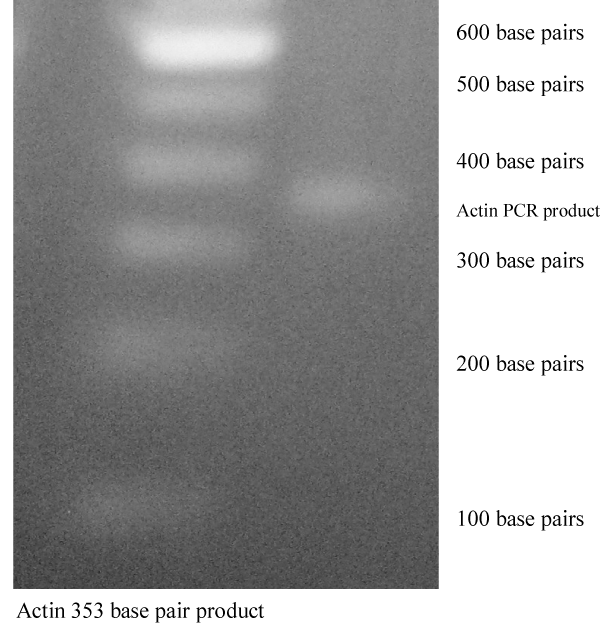





Figure 1
Inhibition of in vitro growth of LNCaP cells by mono- and bispecific oligos.
Figure 1
Inhibition of in vitro growth of LNCaP cells by mono- and bispecific oligos.