Authors:
Andrea Cavallaro1, Giorgio Maria Paolo Graziano2, Marco Cavallaro3 and Antonino Graziano4*
1Surgical and Breast Unit Azienda Policlinico Ct, Italy
2University of Catania, Medical School, Italy
3Radiology unit ASP Ragusa, Italy
4Aggregate Professor, University of Catania Azienda Policlinico Dpt Sciences Surgery and advanced technologies “ G Ingrassia” , via S Sofia 86, Catania. Cap 95125, Italy
Received: 18 September, 2015; Accepted: 27 October, 2015; Published: 28 October, 2015
Prof. Antonino Graziano, 1Aggregate professor, DPT of Surgical Sciences and organ transplants, via S Sofia n 87 Catania cap, 95125 Sicilia Italy, University of Catania, Tel: 0953782880; E-mail:
Cavallaro A, Paolo Graziano GM, Cavallaro M, Graziano A (2015) The Neuroendocrine Cancer. Personal Comments and Operational Remarks. J Surg Surgical Res 1(3): 053-058. 10.17352/2455-2968.000014
© 2015 Cavallaro A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Neuroendocrine tumors; Treatment
Indroduction: Neuroendocrine tumors (NEN) of the gastro-entero-pancreatic tract (GEP) are a group in themselves very heterogeneous of tumors that are different for the site of localization in the digestive tract (foregut, midgut and hindgut), both in relation to the pathological aspects, functional activity and nosographic classification.
Materials and methods: In the period from January 2001 to July 2006 (III Clinical Surgery unit) and from January 2006 to October 2013 (Surgery of the Digestive Tract Unit), UOCS of the University Hospital of Catania has come to our observation 4 patients in the first period and six in the second period. Cases of NEN-GEP we observed allow to highlight how there are elements of clinical differences that make it difficult framing them.
Results: Are summarized personal pathologic and therapeutic results.
Discussion: The interest in the subject stems also from the fact that tumors NEN / GEP, whose detection is now more usual, are malignancies in which it appears increasingly important invasive activity. Despite the huge amount of data acquired, however, several features have yet to be investigated in order to better define the clinical behaviors, develop more precise diagnostic modalities and examine further possibilities for related treatment.
Conclusions: The Authors suggest some recommendations for an appropriate, better treatment of these lesions.
Introduction
The neuroendocrine tumors (NET) are a heterogeneous neoplasms that derive from epithelial cells with neuroendocrine differentiation. The natural history and prognosis varies highlighting the importance of identifying accurate prognosis and predictive biomarkers. Nets can result in a wide array of symptoms based upon the various molecules secreted by the tumor (Oberg K 2010).
They different not only for the site of localization in the digestive tract (foregut, midget and hindgut), both also relatively to pathological aspects, functional activity and nosography classification [1]. The latter figure, with its inaccuracies, makes it difficult to recognize the diagnostic and therapeutic appropriate treatment. A contribution to the problems related to this important chapter of the disease can result from the review of some cases come to our observation that due to undefined and unpredictable behavior, as well as for the anatomical sites of onset, emphasize the need for a continuous review of the topic. The decade of life more common for their detection is the IV [4,6]. The characteristics that most contribute to the difficulties of nosographic framing are the frequent simultaneous involvement of several different cell populations, with the coexistence of so called mixed tumors; the not rare secretion of hormones by the neoplastic tissues (both secretions ortoendocrine but also paraendocrine), secretions sometimes changing spontaneously or in response to drug treatment [3]. Even the variability of prognostic factors in relation to the place of occurrence, the TNM stage, the WHO classification - which compares them to the morphology both mitotic index (Ki67)-, the expression of somatostatin receptors, the age of the patients, make because of the problems that still surround the clinical appearance of these diseases. The finding in the cases we observed and treated to some discrepancies in the clinical behavior of the disease compared to what today is known, leads to underline them in work and suggests that neuroendocrine tumors (NET) are still a class of neoplastic disease, which, in terms of nosography, must always be reconsidered.
Materials and Methods
In the period from January 2001 to July 2006 (III Clinical Surgery unit) and from January 2006 to October 2013 (Surgery of the Digestive Tract Unit), UOCS of the University Hospital of Catania has come to our observation 4 patients in the first period and six in the second period, totally 10 patients. On chronologic order, were found 1) a tumor of the tail of the pancreas, 2) one in the colon, 3) one in the appendix and 4) one in the small intestine; moreover 5)liver metastases spread of unspecified origin, 6) multiple tumors of the stomach, 7)a NEN in the appendix, 8) one in the colon, 9) a NEN of the small intestine in a young woman occluded by volvulus of the ileal loop suffering from Crohn’s disease, 10) a neuroendocrine tumor of the stomach associated with gastric adenocarcinoma. Table 1 shows the clinical cases seen in chronological order and their surgical treatment.
Results
1. 54 year old woman whose parents both died of cancer. This was a clinical picture of recurrent renal colic lefts associated with emaciation, weakness and easy fatigability. The uro-CT showed a solid lesion of 7 cm in diameter which distorted the tail of the pancreas and with its lower pole moved the left corner of the transverse and the ipsilateral ureter. Intervention showed free fluid in the peritoneal cavity (in the absence of positive cytology). It was made a spleno-distal pancreatectomy with omentectomy Microscopic examination: poorly differentiated small cell neuro-endocrine carcinoma. Metastases in 5 of 7 lymph nodes retrieved. Immunohistochemistry: positive vimentin and positive neuron-specific enolase. Negative was instead chromogranin A. Adjuvant chemotherapy. 3y and 5m later peritoneal carcinosis. Exitus.
2. Woman of 56 years with abdominal cramps, with diarrheal discharges (8.10 / day), hot flashes with sweating, hypertensive crisis. Chromogranin serum was high. The 5-hydroxy indole acetic acid in the urine of 24 hours was very high. The octreoscan highlighted a significant accumulation of somatostatin receptors tissue SS-R2, R5 SS-office alleged in the right colon. It was carried out a right hemi-colectomy with ileo-transverse-stoma end-to-side. Repetition octreoscan six months later, would not show any accumulation of radio medicine. The symptoms had disappeared Specimen’s examination: only submucosal lymphoid follicular hypertrophy at the ileal-caecal junction, with nonspecific chronic inflammation and reactive lymphadenitis. Six months later, octreoscan repetition, would not show any accumulation of radio label. The symptoms had disappeared. Today alive and asyntomatic. Disseminated neoplastic neuroendocrine cells?
3. Woman of 30 years with repeated abdominal colic in his right side, accompanied by nausea and vomiting. Appendectomy. Pathologic examination: 10mm well differentiated neuro-endocrine neoplasia in the context of an hyperemic and edematous appendix, with leukocytic infiltrate. Alive.
4. 63 years old woman with abdominal colic in spontaneous remission accompanied by bloating and bowel closed feces and gas. CT revealed a formation near the margin ileal, mesenteric side, richly vascularized approximately 3 cm, assessed as likely leiomyosarcoma, with retraction of the meso and angle of the loop. Resection of ileal loop with its meso. Pathology: solid mass, near the ileal margin, mesenteric side, with retraction of the meso and loop distortion. Histology: well-differentiated neuroendocrine carcinoma of the ileum with metastasis to 2/5 lymph nodes. Adjuvant chemotherapy. Alive at 4y, 4m. Loss to follow up.
5. Woman 61 years with flushing syndrome (diarrhea, flushing, bronchospasm), and dry hands. CT: multiple liver metastases in all segments. Octreoscan: only hepatic lesions, with no signs of the primary lesion. Biopsy: differentiated neuroendocrine carcinoma.Treatment: long-acting octreotide. Relieves of symptoms, accompanied by the disappearance of many repetitive nodules and by the significant decrease in the volume of those residues. Alive at 6y.
6. Woman of 58 years with peripheral neurological disorders (paresthesia) related to chronic atrophic gastritis type Biermer. A gastroscopy noted 3 mucosal lesions including one retracted in the antrum. EUS revealed thickening of parietal antrum and enlarged celiac lymph nodes. Octreoscan negative. In anticipation of surgical treatment, it was planned and carried out an examination DOTATOC which marked only the three gastric lesions and the lymph node. Nothing else to be identified with the method. Given the Demonstrate only exclusive locoregional involvment, it was performed a total gastrostomy with D2 esophagus-jeiunal anastomosis on excluded loop. Pathology: histological confirmation of multiple neuroendocrine well differentiated tumors, N +. It was proposed by the oncologist, according to obvious radical surgery, adjuvant treatment with long-acting somatostatin, without, at least initially, to resort to antiblastic therapy. Alive at 5y 8m.
7. 30 year old man with recurrent poussée pain in the right iliac fossa. Appendectomy. Histology: 6 mm differentiated neuroendocrine tumor cells, enterochromaffin type carcinoid. Parvicellular flogistic infiltration. One mouth postoperative Adjuvant longastatin. Alive and asyntomatic without any therapy, to day.
8. Young woman of 24 years hospitalized for emergency abdominal pain, vomiting and obstipation in feces and gas. Direct abdomen with air-fluid levels. CT described as thickened intestinal loop by vascular changes. It was found at laparotomy a by rotation bowel loop volvulus. It was practiced a resection of the thickened and cyanotic loop with its meso, reconstituing the intestinal continuity with terminal-side ileal anastomosis for discrepancy of the anastomotic stumps. Histology: advanced ischemic sufferance, in a framework of inflammation compatible with Crohn’s disease. Volvulus was likely been caused by the presence of a nodule of poorly differentiated neuroendocrine carcinoma of about 3-4 cm in diameter. Absence of lymph node repetitions. Adjuvant chemotherapy. Multiple hepatic repetition after 5y 2m. Exitus.
9. 40 year old man with frequent repeated abdominal pain. CT described a tumor in the right upper quadrant depending on colon or pancreas. At laparotomy it was found that the tumor, a 1-2cm shortly peduncolated everted neoformation, was located in ascending colonic wall. It was performed a resection of the colon wall repaired with stapling device. Histology: 8 mm lump of differentiated neuroendocrine tumor with negative planting base. Today, alive.
10. 75 year old woman with epigastric subcontinuus nonspecific pain. CT emphasized a gastric cancer. Gastro-duodenoscopy confirmed the lesion, wich at biopsies was a poorly differentiated adenocarcinoma localized in the antrum. Both TAC and EUS believed the tumor hardly clivable from the pancreas, but were not apparent lymphonodal repetitions. We practiced a total gastrectomy with D2 lymphadenectomy and reconstruction with esophagus jejunal anastomosis on excluded loop. Only scarce fibrous adhesions were present between the posterior wall of the gastric antrum and the front wall of the pancreas. Pathology: poorly differentiated adenocarcinoma of the stomach, with minimal (less than two mm square) infiltration of subserosal, N0, M0. At about 3 cm below the cardia, was identified a nodule of 1.5 cm in diameter of neuroendocrine tumor associated to adenocarcinoma. No adjuvant therapy. No signs of relapse after 5y 8m.
On all cases modest perioperative complications. Absent perioperative mortality.
Classification
According the 2012 ENets guideline, NENS are classified on the basis of their site of localization and diagnosis.
Stomach and duodenum
NET G1, NET G2 and NEC, according to the extent of the invasion of the bowel wall and the presence or absence of metastasis. Diagnostic procedures and therapeutic indications suggested may differ according to histological variant identified by biopsies. For more severe forms, it is indicated radical surgical therapy (gastric resection, gastrostomy) with lymphadenectomy.
Colon and rectum
The behavior of these tumors is clinically similar to that of adenocarcinomas of the colon and rectum. The same applies to the therapeutic treatment.
Pancreas
These tumors can be divided into functional and non-functional form. Among the first varieties include gastrinomas, insulinomas, and other various forms of rare incidence. Among the non-functioning are distinguished forms well differentiated (NF - NEN) and those poorly differentiated (NF - NEC), which are further distinguished by the mitotic index and the values of Ki67. Still, the undifferentiated can be sporadic or inherited. Surgical therapy may want to consider the removal of the nodules for well-differentiated forms, as well as spleno distal pancreatectomy or duodenum cephalic pancreatectomy for those poorly differentiated.
Jeiuno Ileum and appendix
The most common forms are carcinoid tumors, which may be responsible for an endocrine syndrome mostly in the case of liver metastases, but rarely even in the absence of the latter. The treatment type is the bowel resection. For appendicular forms, in the presence of invasion of meso appendix, it can be indicated right colectomy.
Liver metastasis
The presence of metastases does not indicate a phase of non-treatability disease. There is ample space for medical treatments, the instrumental therapy embolization (TAE), the chemo embolization (TACE), as well as for the RFA (radiofrequency ablation), for resective therapy, especially about nodules residues after drug treatment, and also for liver transplantation.
General diagnostic and therapeutic
The diagnosis is supported on the determination of serum markers. Radiological and endoscopic procedures, and the use of radio nuclides. The therapy can be medical (SSH Analogs, chemotherapy) or surgical, which cannot be standardized, but it is not different from the surgery of the digestive system.
Discussion
In our experience, there are cases that do not fully obey the note nosography on endocrine tumors of the digestive system (NEN-GEP), while other interesting considerations can be indicative for reconsidering some data. According the Nets classification (2012) we discuss our cases on their localization.
Stomach
Firstly, the gastric NEN Figure 3 (case 6). Of these tumors, are known three types. A) The variety associated with autoimmune chronic atrophic gastritis; B) that associated with Zollinger-Ellison syndrome and C) the sporadic one. The first picture is characterized by multiple small lesions, with polypoid appearance; gastrin is high, chromogranin A is high too, the Ki67 is very modest, around 2%. Metastases are present only in about 2-5% of cases at diagnosis. The heterozygosity 11q13 is found in 17-73% of cases. The second picture is associated with Zollinger-Ellison syndrome in the context of a MEN of the first type, consisting of hyperthyroidism, pituitary tumor and pancreatic GEP-NEN (S. of Wermer). Gastrin serum level is high, is elevated the chromogranin A, and is low Ki67, at around 2%. Metastases were found around 35% of cases at diagnosis. The heterozygosity is constant. The third picture is characterized by solitary lesions, larger, ulcerated and infiltrating, poorly differentiated. Ki67 is high, metastases are found in more than 50% of cases at diagnosis. The gastrin serum level is normal. There is an atypical carcinoid syndrome with itching, bronchospasm and cutaneous flushing, by histamine release. Heterozygosity is present in 25-50% of cases. Now, the case occurred to our observation presents undeniable elements of deviations from the officially adopted and followed classification. The case studied, being related to chronic atrophic gastritis type A, have to be classified as belonging to the first type of gastric NEN, and is therefore anomalous that may already presents to an incidental diagnosis with lymph node metastases so evident and so important as to require an intervention of gastrectomy with D2 lymphadenectomy. We obviously refer to a real neuroendocrine carcinoma (NEC). It becomes so unattractive S. Rappel’s claim that 99% of patients with gastric NEN of the first type will survive to the extent of 99% after 5 years. In our experience, the CT / PET DOTATOC allowed, beyond the negativity of otreoscan, a careful assessment of the real extent of disease, since impairment M staging would make not feasible surgery with radical intent. IT is therefore undeniable that it is necessary to assess the true extent of the disease, subjecting patients to the PET / CT DOTATOC because the examination can reveal the extent of regional or distant localization of the original NEN and target the correct therapeutic treatment, avoiding over and under treatment. Case 10 is an example of non-rare associations adenocarcinomas-NEN / GEP. Yet the case lends itself to some considerations, especially since the lesion, not detected in the gastric mucosa, was not identified preoperatively by the endoscopist, probably for the relevance of the main antral stenotic lesion. Now, since the elderly patient, the surgeon could have chosen to practice a distal gastric resection (Billroth II) to treat the adenocarcinoma, and therefore unknowingly leave neuroendocrine residual lesion in the gastric stump, opening the door for medico-legal debats and exposing the patient at the need of a reintervention [7,12,22,24].
Colon
Equally strange is the assessment of the case of NEN with colic localization (case 2), who seemed to have all the trappings of the neuroendocrine carcinoid type tumor, with probably ortho or paraendocrine secretion. Is unusual preoperative positive and postoperative negative octreoscan findings, while histological examination of the resected piece only showed follicular lymphoid hyperplasia of the submucosa at the ileum-caecal valve, with nonspecific chronic inflammation and reactive lymphadenitis? Although the combination of hypertensive crisis during episodes of symptoms could evoke the presence of a NEN supported by pheochromocytoma, disappearing after surgery of clinical phenomenology, accompanied by octreoscan negativity, excludes this hypothesis and still makes the little definable clinical case observed. It is likely that the case is one of those, and are not rare, in which rather than a detectable mono-centric neoplasm, there is a form of not localized, cell disseminated tumor, which accounts for about 20% cases in colorectal NEN, especially at the level of the ciecum [2,7,12,22,24]. This case remain otherwise doubtful. Case 9 ( 8 mm nen on a specimen with a lesion of 1-2 cm with free planting base) was simply treated by tangential resection of colonic wall. The conservative resection is justified by the size of the tumor as well as the occasional postoperative finding. The case has been closely monitored over time, to date dotatoc is still negative and the patient is alive and asymptomatic (Figure 1).
-
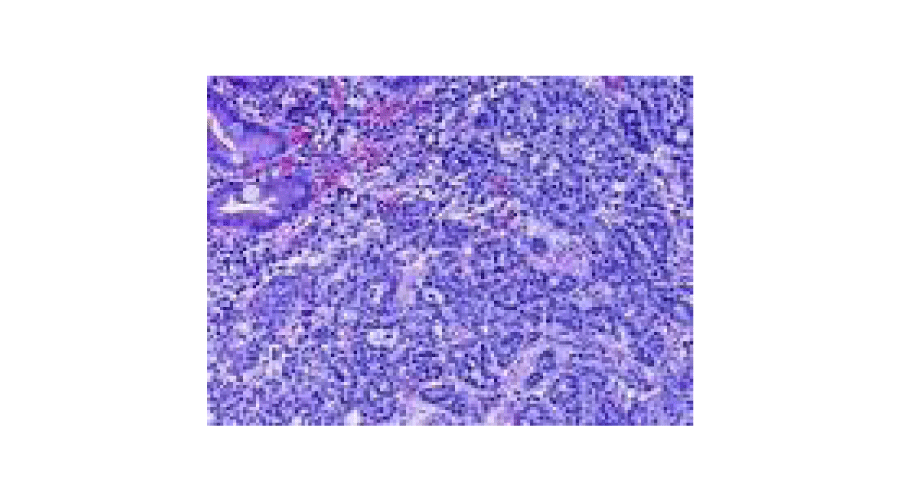
Figure 1:
Colon.
Pancreas
No abnormalities in the clinical assessment and pathologic aspect of this tumor [3,5,8,23]. Case 1 lesion of 7 cm in diameter which distorted the tail of the pancreas. Poorly differentiated small cell neuro- endocrine carcinoma. Metastases in 5 of 7 lymph nodes. Negative was instead chromogranin A. This finding calls into question the close relationship between NEN and A chromogranin.
Ileal
(Case 8) To underlines the strange association, probably occasional, between NET and Crohn disease. Prevedible, unstead (cases 4, 8), the association with stranglement intestinal occlusion, encountered in both cases [3,7,9,10,21]
Appendix
In both cases of our experience was not present an invasion of the meso appendix: case 7, tumor 6 mm differentiated neuroendocrine tumor cells enterochromaffin type carcinoid,flogistic infiltration, case 3, tumor 10 mm in the context of an hyperemic and edematous mucosa [21,22,24] (Figure 2).
-
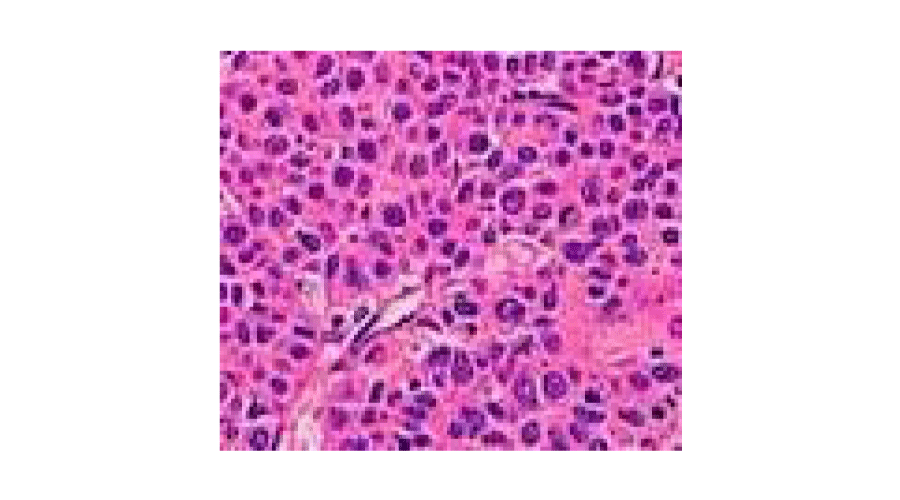
Figure 2:
Appendix.
-
Figure 3:
Appendix.
Liver metastasis
In the case of patient with liver metastases (case 5), the use of PET-CT with 68Gallio – at time not useful - would certainly have allowed the identification of the primary site, not highlighted by the TAC and by octreoscan. In fact the spread of repetitive liver injury did not allow other different treatment choices over the longastatin. However, given that the drugs had extensively so reduced the number of injuries to confine them in one or two sectors, the knowledge of the primary site could have to consider a more aggressive treatment option for removal of the primary lesion associated with liver resection surgery [4,13,15].
Finally in our serie are not present cases of localized rectal NEN that alone should give an account for more than half of the cases of colorectal NEN / GEP encountered. Sure the cases observed, a total of 10 including 2 in the colon, are not many, but certainly when you consider the overall incidence of NEN are not even a few, and it is strange that it had found in our cases not even a case of localized rectal NEN. Perhaps this data should be reconsidered, because the observed prevalence of rectal lesions is, in the review of the 2003 of I.M. Modlin et al. [2,3], relative to U.S.A. patients, referred to colored people or of asian origin. The experience, therefore, to find more cases of NEN in the stomach and in the colon may be in agreement with the consideration of the higher turn-over of cells of the gastric and colonic mucosa, compared to other sections of the digestive system, such as the rectum. To remember that the neuroendocrine appendicular neoplasms are not considered invasive even when close to the muscularis propria (as well as adenocarcinomas, for the absence of lymphatic vessels in the intestinal mucosa, are indicated by the pathologist as dysplasias when not reach the submucosa).
Conclusion
Cases of NEN-GEP we observed allow to highlight how there are elements of clinical differences that make it difficult framing them. The neuro-endocrine repetitive tumors of the liver (patient case 5) remained the only injury displayed by diagnostic imaging and by octotreoscan, which, not allowing you to identify the primary site of NEN neoplasm, has in part influenced treatment decisions for the sole use of somatostatin analogues, limiting the possibilities of surgical options. Even the case (case 6), revealed, despite the occasional diagnosis, that the patient was already in a state of disease boundless from the stomach to the regional nodal level, although the case-like disease in relation to chronic atrophic gastritis type Biemer, should only rarely involve the lymph nodes or distant organs. The case, however, has shown that the exact definition (regional nodes) of the stage of diseas, CT / PET DOTATOC, allowed to adopt a therapeutic approach suitable to the local-regional control of neoplastic disease (D2 gastrectomy), leaving or creating space for more other therapeutic lines, if necessary. Completely abnormal then the clinical and scintigraphic findings of case 2, in which the octreoscan showed an accumulation of the radionuclide at the site of the alleged right colon, with the disappearance of the clinical and scintigraphic data after surgery, but with a simple istopathological follicular hyperplasia at the ileo-ciecal valve in the piece removed. Not then had histological confirmation of NEN, yet probably present in disseminated multicellular form. Even the case 10 reminds us not rare association of NEN / GEP with typical digestive adeno-carcinomas and the importance of its detection to prevent that a resection aimed at removing the most obvious pathology, can however leave in situ other disease with a variable degree of malignancy. Without saying finally the strange absence of rectal localization of NEN / GEP in while not large series, when these locations should constitute about half of the cases of colorectal NEN encountered. Certainly this chapter of pathology, so varied and so complex, no doubt needs further study. The interest in the subject stems also from the fact that tumors NEN / GEP, whose detection is now more usual, are malignancies in which it appears increasingly important invasive activity. Despite the huge amount of data acquired, therefore, several features have yet to be investigated in order to better define the clinical behaviors, develop more precise diagnostic modalities and examine further possibilities for related treatment. Would certainly be appropriate to adopt a cancer registry specifically dedicated to reaching a more documented nosography. In light of the data most recently acquired and the results of our experiences, we should suggest some recommendations: 1) be aware of the true incidence of these neoplasms and their increasingly common finding; 2) use in diagnostic and staging methods CT / PET scintigraphic 68Ga; 3) pay great attention to the existence of associated forms of neuroendocrine tumors with malignant epithelial neoplasms of the digestive system; 4) take into account the possibility of scattered multicellular forms, not one nodular; 5) justify recourse to a radical surgery with strong potential malignancy of these tumors.
-
-
- Crentzfeld W (1994) Historical background and natural history of carcinoids. Digestion 55: 3-10.
- Modlin IM, Lye KD, Kidd M (2003) A 5-decade analysis of 13.715 carcinoid tumors. Cancer 97: 934-959.
- Jordan PH (1999) A personal experience with pancreatic and duodenal neuroendocrine Tumors. Am Coll of Surgeons 189: 470-482 .
- Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, et al. (2000) Endoscopic Ultrasound,Is Highly Accurate and Direct Management in Patients With Neuroendocrine Tumors of the Pancreas. The Am J gastroenterol 95: 2271-2277.
- Ahrendt SA, Komorowski RA, Demeure MJ, Wilson SD, Pitt HA, et al. (2002) Cystic Pancreatic Neuroendocrine Tumors: Is preoperative Diagnosis Possible? J Gastrointest Surg 6: 66-74.
- Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, et al. (2003) Surgical Treatment of Neuroendocrine Metastases to the liver A plea for resection to increase survival. Am Coll of surgeons 197: 29-37 .
- Bordi C, D’adda T, Pizzi S, Crafa P, Rindi G (2002) The assement of malignancy in endocrine tumors of the gastrointestinal tract. Current Diagnostic Pathology 8: 421-429.
- Secil M, Goktay AY, Oksuzler Y, Sagol O, Dicle O, et al. (2002) CT findings of non-functioning neuroendocrine pancreatic tumors. Comput Med Imaging Graph 26: 43-45 .
- Gandolfi L, Torresan F, Solmi L, Puccetti A (2003) The role of ultrasound in biliary and pancreatic disea se 16: 141-159.
- Graziano A (2007) J pouch colon anal reconstruction after anterior resection for cancer. ACTA CHIRURGICA MEDITERRANEA 23: 97-100.
- Christ E, Wild D, Forrer F, Brändle M, Sahli R, et al. (2009) Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab 94: 4398–4405 .
- R Graziani, A Brandalise, M Bellotti, R Manfredi, A Contro, M Falconi, L. Boninsegna, R. Pozzi Mucelli (2010) Imaging of neuroendocrine gastroenteropancreatic tumours. European J of Ultrasound radiologia medica 115: 1047-1064 .
- Kim SJ, Kim JW, Han SW, Oh DY, Lee SH, et al. (2010) Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: tumor grade and metastatic site are important for treatment strategy. BMC Cancer 10: 448.
- Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, et al. (2010) Metastatic tumors in the pancreas in the modern era. J Am Coll Surg 211: 749–753 .
- de Herder WW, Mazzaferro V, Tavecchio L, Wiedenmann B (2010) Multidisciplinary approach for the treatment of neuroendocrine tumors. Tumori 96: 833–846 .
- Sidéris L, Dubé P, Rinke A (2012) Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist 17: 747–755 .
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, et al. (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364: 501–513 .
- Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, et al. (2011) First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 117: 268–275 .
- Oberstein PE, Saif MW (2012) Update on novel therapies for pancreatic neuroendocrine tumors. JOP 13: 372–375 .
- Kvols LK, Oberg KE, O'Dorisio TM, Mohideen P, de Herder WW, et al. (2012) Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a Phase II study. Endocr Relat Cancer 19: 657–666 .
- (2013) guidelines AIOM 2013 XV national Congress Rome 23-25.
- Cavallaro A, Lauretta A, Cavallaro M, Pennisi S, Cavallaro V (2006) Surgery on gastrointestinal stromal tumor CD117+ (G.I.S.T.): Personal experience Ann Ital Chir 77: 137-141 .
- Mauriello C, Napolitano S, Gambardella C, Candela G, De Vita F, et al. (2015) Conservative management and parenchyma-sparing resections of pancreatic neuroendocrine tumors: Literature review. Int J Surg pii: S10-S14 .
- ENETS Guidelines, TNM Grading (2012) Standards of Care and Metastases The 2012 ENETS Consensus Guidelines for the Diagnosis and Treatment of Neuroendocrine Tumors are as follows: Neuroendocrinology 95: 74–87.









Table 1:
Clinical cases seen in chronological order and their surgical treatment.